
Welcome to this video tutorial on acid-base balance and blood gas interpretation.
pH and Acid-Base Homeostasis
Acid-base balance is critical in maintaining homeostasis in the body. Acids are substances that can give up a hydrogen ion and bases are substances that can accept a hydrogen ion. Acidity or alkalinity of body fluids is expressed in terms of the concentration of hydrogen ions, or pH.
Hydrogen ions have an inverse relationship to the pH. More H+ ions cause a lower pH, which is acidic; fewer H+ ions cause a higher pH, which is alkaline. The accumulation of acid in the blood or a loss of base causes acidosis. The accumulation of base in the blood or a loss of acid causes alkalosis.
The primary factors in the homeostatic process include the lungs, kidneys, and the buffer system. The lungs control certain amounts of carbon dioxide in the blood; therefore, if the lungs are not functioning properly, CO2 builds up, causing increased carbonic acid, which affects blood pH and leads to acidosis. The main function of the kidneys is retaining or excreting bicarbonate (HCO3), which is what neutralizes the excess acid in the blood.
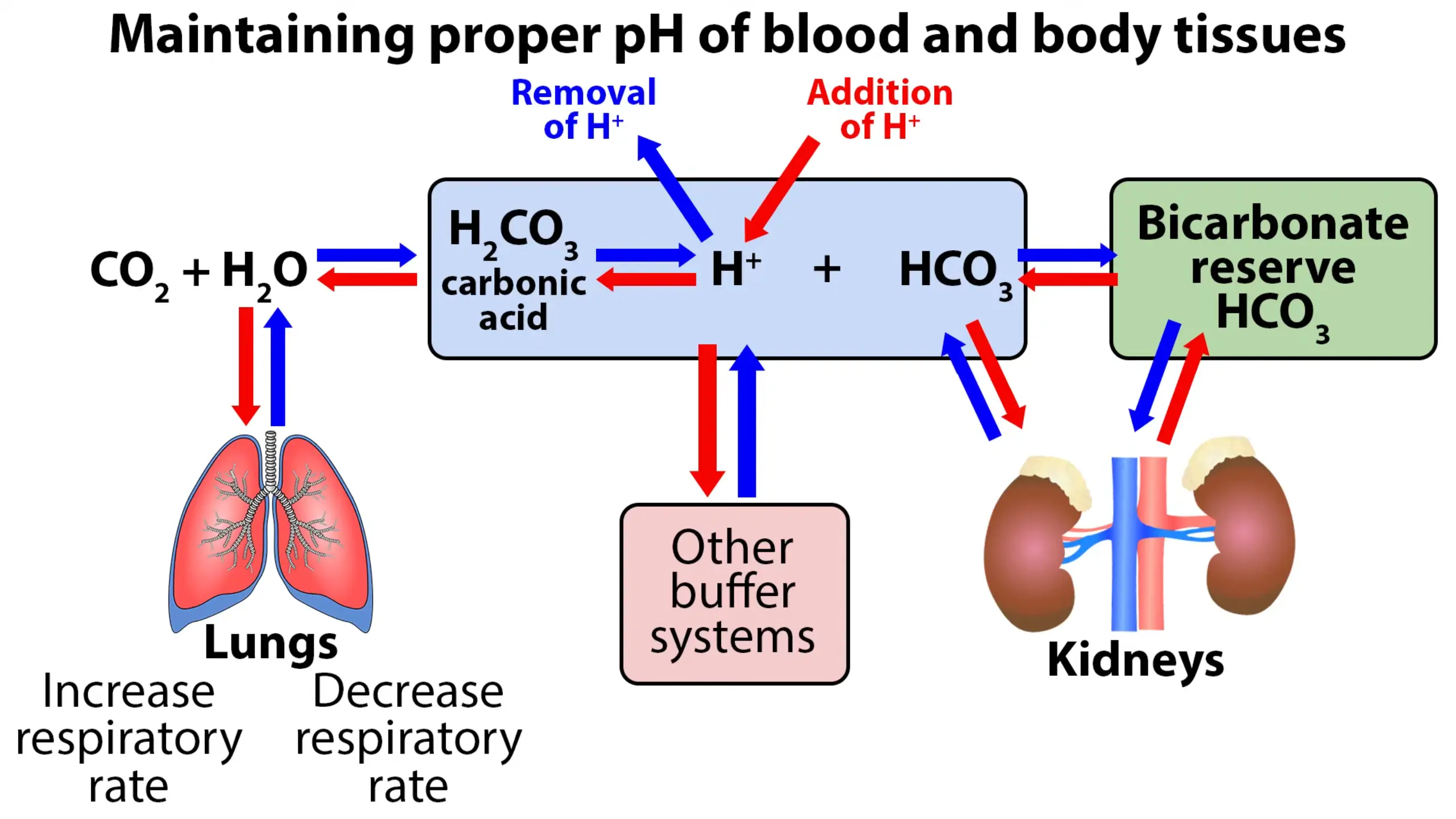
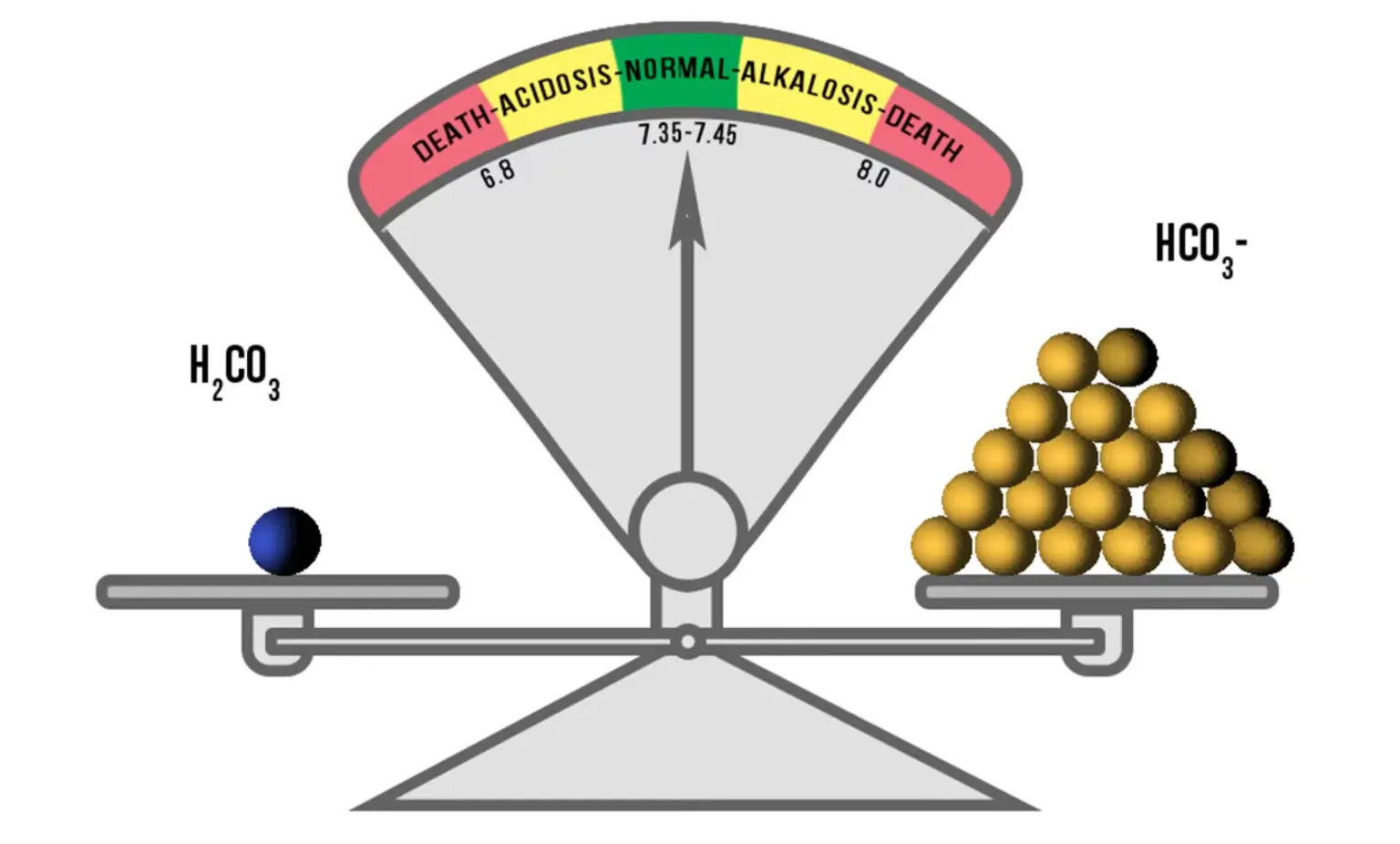
If both the lungs & kidneys are working properly, the natural buildup of acids will be neutralized effectively by the buffer system. The buffer system works very quickly to maintain proper pH of the blood and body tissues. The bicarbonate will neutralize the correct amount of carbonic acid molecules to maintain the correct ratio of 20:1 acid molecules. This ratio of 20 bicarbonate (HCO3) to 1 carbonic acid (H2CO3) will keep the blood pH in the range of 7.35 to 7.45.
Analyzing and Interpreting ABG Results
The arterial blood gas (ABG) provides valuable information about the acid-base balance of the blood and the oxygenation status of the blood. It measures the arterial pH, PaCO2, bicarbonate, PaO2, oxygen saturation, and base excess.
Arterial blood is used because venous blood is not suitable for the assessment of oxygen tension and pH. The nurse draws blood from either the radial, brachial, or femoral artery; or from an arterial line. The radial artery is most commonly used because it is accessible, easily positioned, and more comfortable for the patient.
The sample should be drawn with a heparinized syringe, without exposure to air. The sample is then placed on ice (which slows the clotting of blood), and rushed to the lab for analysis.
When analyzing ABG’s, there are several normal values that need to be memorized. When assessing acid-base status, the most important component to look at first is the pH. 7.4 is the perfect pH, with a normal range of 7.35-7.45. PaCO2 (partial pressure of carbon dioxide) reflects alveolar function and ranges from 35-45 mmHg. Bicarbonate (HCO3), the major base component, has a normal value of 22 to 26 mEq/L. The total concentration of bicarbonate in the blood may be referred to as serum carbon dioxide, serum bicarbonate, and total carbon dioxide content.
When you need to interpret ABGs, it is helpful to lay out a chart, with pH at the top of the first column, then lungs (PaCO2) and kidneys (HCO3). Start labeling in the second column—acid, base, acid—continuing on to the 3rd column—base, acid, base. And if you memorize the normal values, you can plug them right in – pH 7.35-7.45, PaCO2 35-45, HCO3 22-26.
With a chart written out, you can easily figure out ABG problems. Let’s go over a few examples.
If the pH is 7.29 (it’s less than 7.35), it is acidic. The PaCO2 is 50 (greater than 45), so it is acidic. The HCO3 is 24, which is normal. Since the pH is acidotic and the CO2 is acidotic, we know the acid-base disturbance is being caused by the respiratory system, and is called respiratory acidosis.
Since PaCO2 is high and represents lung function, it tells us there is excess CO2 retention from hypoventilation or CNS depression.
In our next example, the pH is 7.5, it is basic, so circle ‘base.’ The PaCO2 is 33, it is also basic. The HCO3 is 25, which is within normal range. In this case, there is excess CO2 being excreted, possibly caused by hyperventilation or fever. The acid-base imbalance is again caused by the respiratory system, but with an alkalotic pH, meaning it would be respiratory alkalosis.
Ok, now we will look at metabolic situations. Metabolic acidosis and metabolic alkalosis are caused by the levels of bicarbonate ion in the blood. The kidneys excrete these ions into the urine so they can be eliminated when not needed. If the pH is low, in this example 7.28, circle ‘acid.’ The PaCO2 is within normal range at 40, and the HCO3 is acidic at 20. With low bicarb levels and a low pH, you have metabolic acidosis.
In our next example, the pH is 7.51, so it is basic. The PaCO2 is 39, within normal range. The bicarb level is above 26, so it is high, also basic. This means we have metabolic alkalosis. Metabolic alkalosis can result from inadequate excretion of acids due to renal disease, loss of potassium from diuretic therapy, and other causes.
Ok, we’ve looked at how the levels of CO2 and HCO3 can alter the blood pH. However, the body does try to bring the pH back to normal by compensating for the imbalances.
For example, the pH is acidotic, the CO2 is alkalotic, and the HCO3 is acidotic. Both the HCO3 and pH are acidotic, making the primary acid-base disorder metabolic acidosis. The CO3 is opposite of the pH and would be evidence of compensation from the respiratory system.
Another way to remember acid – base imbalances is with the mnemonic ROME. Respiratory Opposite, Metabolic Equal. We know respiratory involves CO2, and CO2 will be going in the opposite direction of the pH. The pH is low (acidotic), the CO2 will be high (also acidotic) – resulting in respiratory acidosis. When the pH is high (alkalotic), and the CO2 is low (also alkalotic)—we have respiratory alkalosis.
We know metabolic involves the bicarbonate ion (HCO3). When the pH is low (acidotic) and the HCO3 is low (also acidotic), the result is metabolic acidosis. If the pH is high (alkalotic), and the HCO3 is also high (also alkalotic)—we have metabolic alkalosis.
Review
Let’s go over a review.
Look at the pH 1st. If it’s low, it indicates acidosis. If it’s high, it indicates alkalosis.
Next, look at the CO2. The respiratory system controls the CO2. Determine if it’s higher than 45 or lower than 35.
Next, look at the HCO3, which is controlled by the kidneys. Is it less than 22 or greater than 26? Determine if there is any compensation present.
It is also important to know the oxygenation status of a patient. The partial pressure of arterial oxygen is the amount of oxygen that is dissolved in the blood. It is referred to as PaO2 and is normally 80-100 mmHg. The PaO2 does not play a role in acid-base regulation, however, low PaO2 indicates an alteration in respiratory function or breathing air with a low level of oxygen.
Oxygen is also combined with hemoglobin. The SaO2 (oxygen saturation of hemoglobin) is expressed as a percentage. At full saturation, the normal SaO2 is 95-100%. The SaO2 and PaO2 are directly related to each other—as one increases, the other usually increases also. So, oxygen is present in the blood in two forms: oxygen dissolved in the blood and oxygen combined with hemoglobin. If they are below normal, there is evidence of hypoxemia.
Thank you for watching this video on acid-base balance and blood gas interpretation!
