
Hey there! Welcome to this Mometrix video over one of the most basic types of bonds: covalent bonds!
What is a Covalent Bond?
A covalent bond is a type of chemical bonding that refers to the sharing of valence electrons between atoms in an attempt to become stable. Unlike ionic bonds where atoms gain or lose electrons, covalent bonds involve the sharing of electrons.
Here is another way to remember it: co means “share” and valent means “valence.” So, we get “the sharing of valence electrons.”
The Octet Rule and Electronegativity
Atoms just want to be stable like the noble gases in the world, which means they have to achieve the octet rule, meaning they need 8 electrons in their outer shell (valence electrons). Atoms that have a similar electronegativity tend to bond covalently.
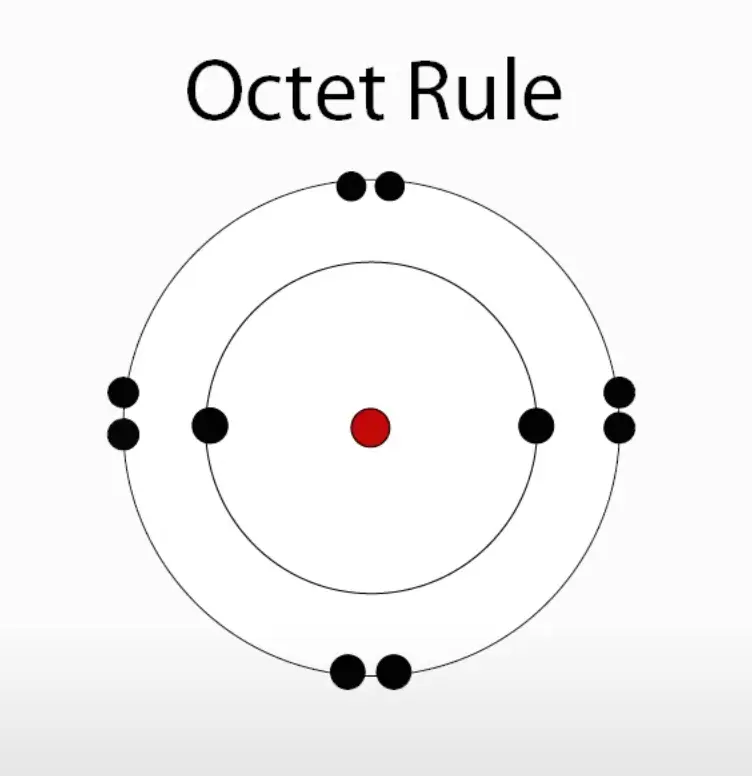
Single Bonds and Double Bonds
For instance, chlorine atoms are highly electronegative with 7 electrons, and just one away from achieving the octet rule. So, when you get two chlorine atoms they are going to say to one another “This for sure is not going to be an ionic relationship. You aren’t getting any of these electrons! I’m one away from having an octet, I’m not givin’ up nothin’.”
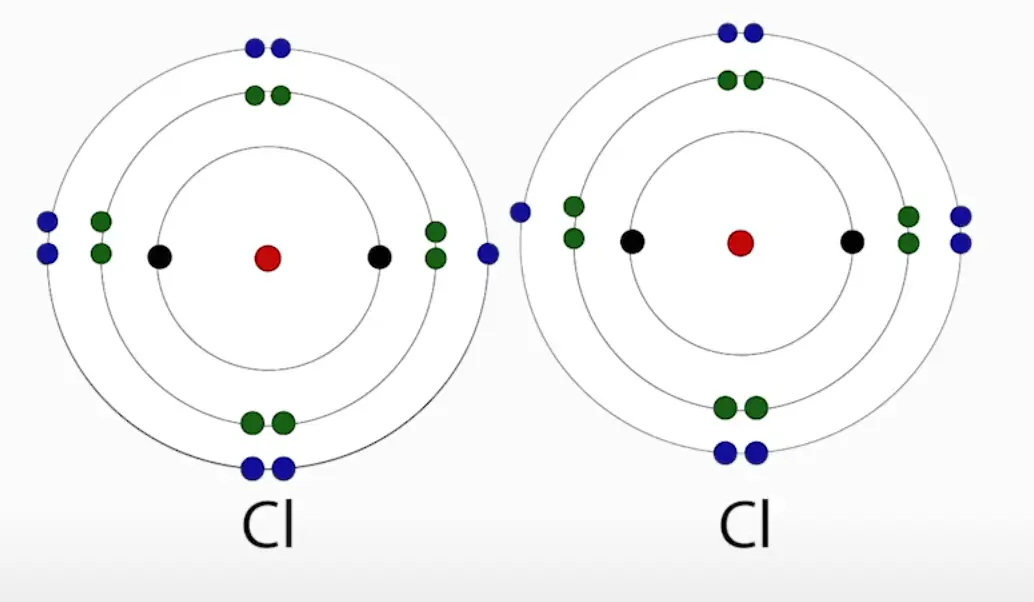
So, in this case, the solution is to compromise and share, because they are more stable together than apart. Because they each are sharing one electron, this is called a single bond. However, atoms can share multiple electrons, like oxygen.
Oxygen atoms also have a high electronegativity with 2 unpaired electrons; so, when an oxygen atom crosses the path of another oxygen atom, then they are going to double bond, and thus satisfying the octet rule.
Notation for Covalent Bonds
Another helpful tool to know is the notation for single-bond and double-bond. If you don’t know it you may think that you are being asked to subtract a chlorine from a chlorine, or that an oxygen atom (O) equals an oxygen atom. But, when you see one dash between two chlorine molecules this signifies that a single bond has taken place, and when you see two dashes, looking like an equal sign, this signifies that a double bond has taken place.
Versatility
Covalent bonds are awesome, because they allow for a vast variety of compounds, much more than ionic bonds. Because of covalent bonding we have things like water (H2O), carbon dioxide (CO2), the kind of alcohol that people drink (C2H5OH), and even larger compounds like aspirin, estrogen, and testosterone. All proteins in your body are made up of hundreds, thousands, and even millions of atoms all covalently bonded. Another cool thing to note is that all of your larger compounds are going to be crawling with carbon atoms, because carbon is able to continually build on itself.
Covalent bonds are one of the most simple yet intrinsic gifts.
I hope this was helpful! See you next time!