
Hi, and welcome to this video on the difference between rocks and minerals! In this video, we’ll learn what rocks and minerals actually are and look at several different types as we talk about the differences between them. Let’s get started!
So, what’s the difference between rocks and minerals? In short, rocks are made up of a variety of different materials, including some minerals. The amount of mineral that’s in a rock will vary depending on what rock you’re looking at. For example, granite is made mostly of the minerals quartz and feldspar. On the other hand, a soft sedimentary rock like limestone might contain traces of pyrite but is not primarily composed of that mineral.
Minerals
A mineral is a solid, inorganic, naturally occurring, ordered arrangement of an element or compound that has a fairly specific composition. When I say, “ordered arrangement of an element or compound,” I mean it has a crystalline structure. This internal crystalline structure repeats indefinitely. This means that if you had a piece of a mineral, and smashed it with a hammer, all the tiny pieces that broke off will have the same internal crystalline structure as the larger piece:

No matter how small you make that piece of the mineral, it will always retain its crystalline structure. A great example of this is halite, which you might know better as rock salt.
The bonds between sodium ions, Na+, and chloride ions, Cl-, always arrange themselves in this very specific way. This crystalline structure forms halite. The bonds here are electrostatic, meaning there is an attraction between the positively charged sodium cations and the negatively charged chloride anions.
A mineral example of this would be a diamond, which is a very specific arrangement of carbon atoms.
Speaking of diamonds, let’s take a quick look at polymorphs. Polymorphs are the same compound, same composition, but with different crystalline structures. Diamond, the hardest naturally occurring mineral, is used for cutting and polishing and has a three-dimensional compact framework of specifically arranged carbon atoms.
Graphite, the stuff inside the pencils you write with, is also made of a specific arrangement of carbon atoms. So if diamonds and graphite are made of the same thing, why is graphite so soft, while diamonds are so hard? Well, take a look at the difference between the two structures:
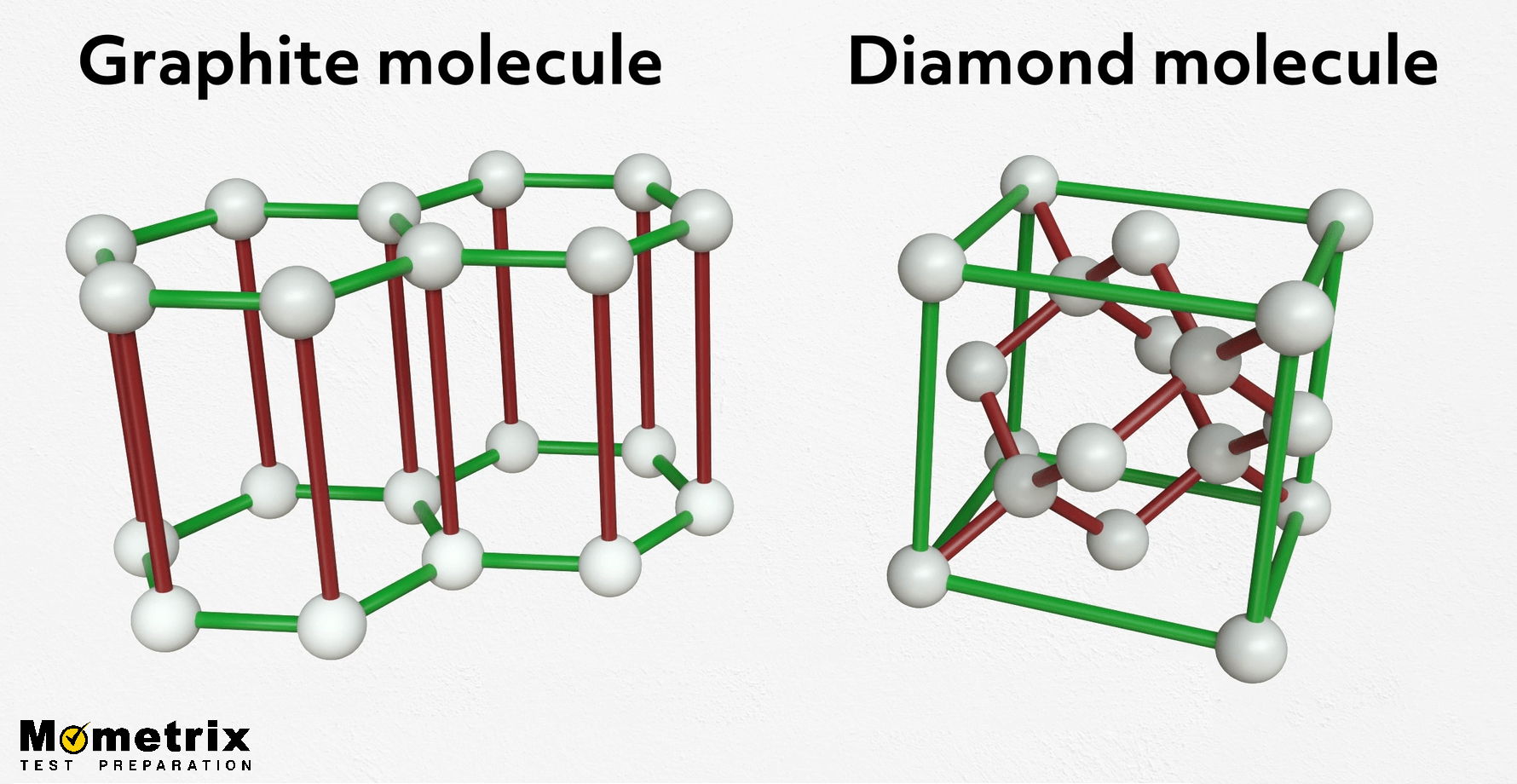
Even though they’re made of the same thing, their structures are pretty different. Graphite consists of sheets of carbon atoms instead of the more robust cube shape we see in diamond’s structure. The atoms are strongly bonded within the sheets but are very weak between the layers.
Sometimes there are compositional variations in minerals, where ions of similar size can switch with one another without disrupting the mineral’s internal framework. These are known as solid solution materials. For example, the mineral olivine has two different variations: fayalite and forsterite.
See how the iron and magnesium are interchangeable? This is an example of a solid solution.
There are also things called mineraloids, which are naturally occurring geologic materials, but they do not have a crystalline structure. They may look like minerals, but don’t be fooled – since there is no crystalline structure, they are not minerals.
An example of a mineraloid is obsidian, which is volcanic glass. If you took a piece of obsidian and smashed it with a hammer, you would see it does not break on the same planes and retain its crystalline structure (because it doesn’t have one).
Minerals are generally classified by their anions or anionic group. But more broadly, they are classified as silicate or non-silicate minerals, with silicate minerals being the most common.
Silicate Minerals
The silicate minerals, made from silicon and oxygen, have five basic structures:
- Independent tetrahedra
- Single chain
- Double chain
- Sheet
- 3D framework
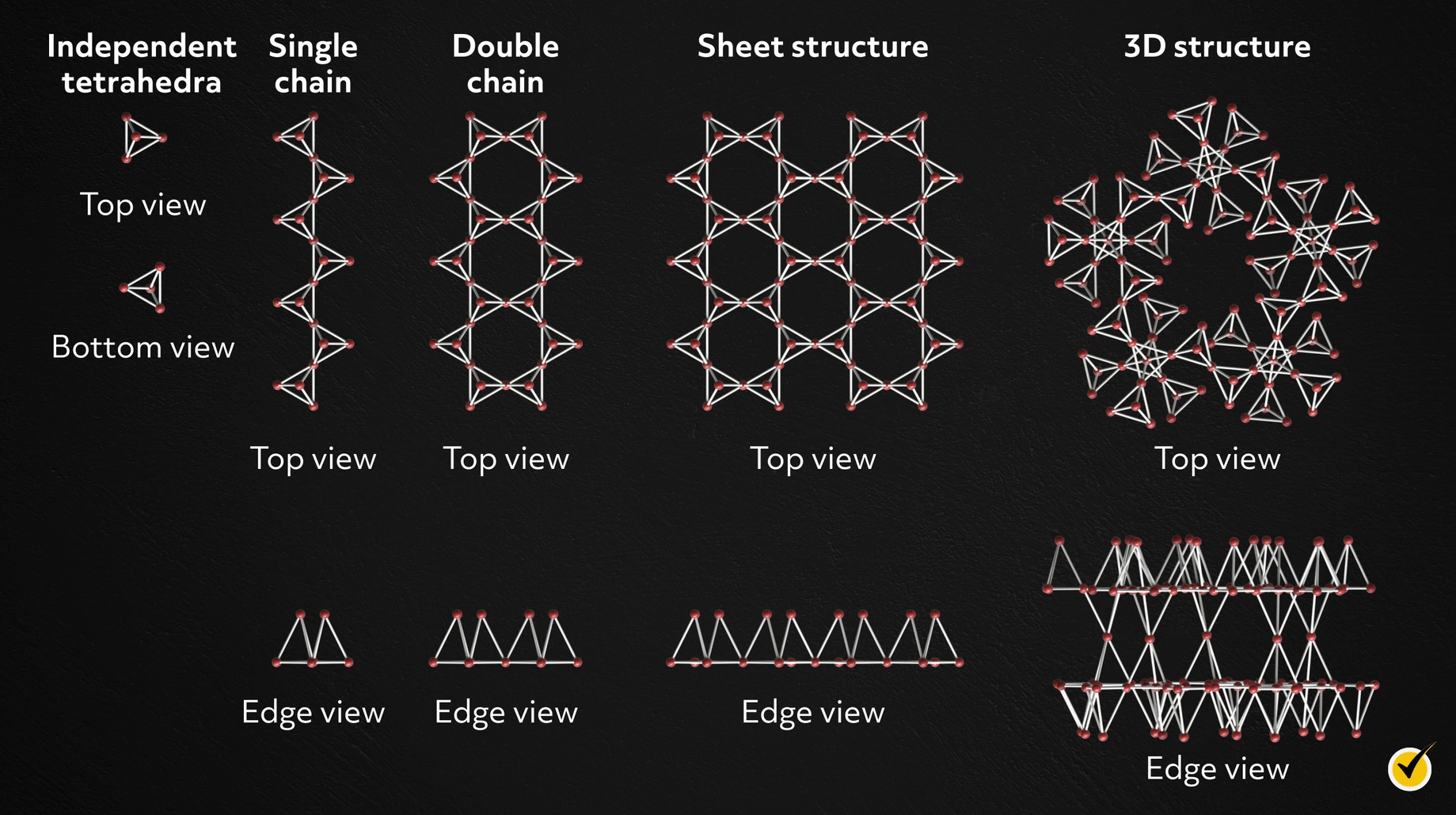
Silicate minerals include olivine, quartz, chert, feldspar, mica, and kaolinite. Most rock-forming minerals are silicates, and the feldspar group is most abundant in the Earth’s crust.
Non-silicate Minerals
The non-silicate minerals can be further classified into carbonates, oxides, halides, sulfates, sulfides, and native minerals. The carbonate group contains carbon and oxygen bonded with another element. The oxide group contains oxygen bonded with metal. The halide group contains chlorine or fluorine, typically bonded with a metal from the left side of the periodic table. The sulfate group contains sulfur combined with oxygen and bonded to a metal. The sulfide group contains sulfur bonded with metal.
Native minerals simply contain a single element.
Let’s switch gears and talk about rocks.
Rocks
There are three types of rocks: igneous, sedimentary, and metamorphic. Most of the earth’s crust is made of igneous rock. Do you remember what type of minerals are primarily found in the earth’s crust? Silicate minerals, which means that igneous rocks are made up of silicate minerals.
Igneous Rocks
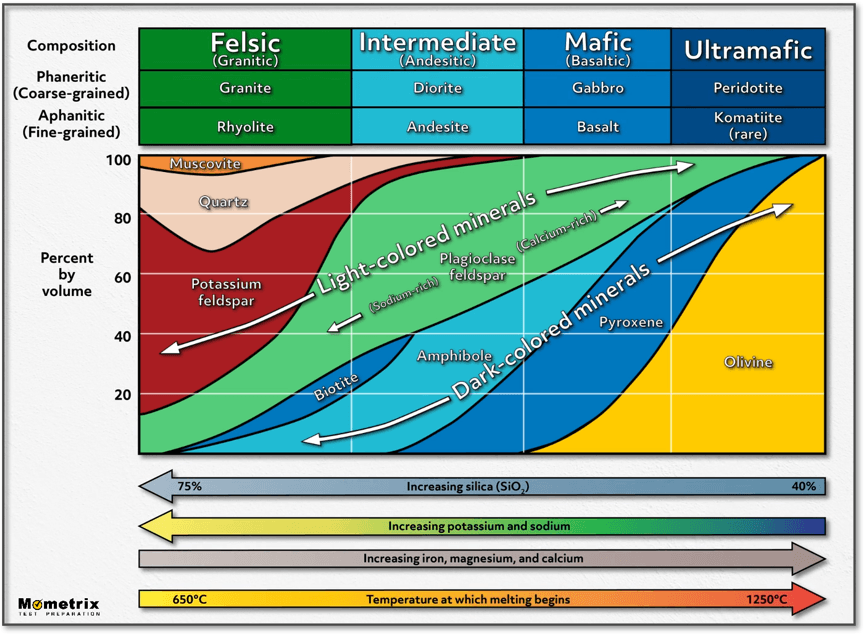
Igneous rocks can be dark or light. Igneous rocks that are rich in iron or magnesium are also low in silica and tend to be dark. Igneous rocks that contain more potassium, sodium, or calcium instead of iron or magnesium are higher in silica and tend to be lighter. There are four types of igneous rocks that can be ranked by their composition: felsic, which are lightest in color and highest in silica; intermediate; mafic; and ultramafic, which are darkest in color and lowest in silica.
Sedimentary Rocks
Just as igneous rocks can be further classified by their mineral composition, so can sedimentary rocks.
Detrital sedimentary rocks are composed of clay minerals, quartz, feldspars, and micas. They are formed from sediments that have been weathered and transported by the environment. These types of rocks are generally distinguished by their particle size.
Chemical sedimentary rocks can be made from minerals like calcite, aragonite, and different varieties of chert. These rocks are formed from precipitated material that was once in a solution; either through an inorganic process like evaporation or through organic processes carried out by water-dwelling organisms.
Metamorphic Rocks
You probably get the picture by now—metamorphic rocks can also be classified based on the minerals which they contain. In this case, though, it’s a little different. Because metamorphic rocks form due to the increased temperature and/or pressure of another type of rock, each metamorphic rock will have a “parent” rock.
For example, quartzite is a metamorphic rock, and its parent rock is quartz, which is a silicate mineral. Marble is a metamorphic rock, and its parent rock is limestone, which is a non-silicate mineral in the carbonate group.
Review
Okay, now that we’ve covered all the details of rocks and minerals, let’s go over a couple of review questions to see what you remember!
1. Which one of the following is not a definitive characteristic of a mineral?
- Solid
- Naturally occurring
- Organic
- Crystalline structure
2. Which of the following is not a type of igneous rock?
- Silicate
- Felsic
- Mafic
- B & C
- A & B
I hope this review was helpful! Thanks for watching, and happy studying!
