
Hi, and welcome to this video on electric charge! In this video, we’re going to look at what electric charge is and how it’s measured. Let’s get started!
Fundamental Properties
Electric charge is a fundamental property of matter. All physical things in the universe are made up of matter, all matter is made up of atoms, and atoms are made up of particles. These particles can be positively charged, negatively charged, or neutral. Protons are positively charged, electrons are negatively charged, and neutrons have no charge at all, making them neutral.
Additivity and Net Charge
One property of electric charge is that it is additive. This means that when multiple charges of the same kind are put together, they add up to one big charge, be it positive or negative. And if we have a mixture of positive and negative charges, we can find the net charge of the overall mixture.
For example, if we have an object made up of 14 protons, which are positive, and 12 electrons, which are negative, then we end up with a net charge of positive 2. We simply add 1 for each proton and subtract 1 for each electron. If the resulting number is positive, we have a net positive charge and if the resulting number is negative, we’ll have a net negative charge. Note that neutral particles will make no difference in the net charge.
Electric Force and Interactions
It’s also important to understand the interaction between charged particles. All electrically charged particles exert a force on all other electrically charged particles, aptly named the electric force. The electric force is described by this equation:
The letter \(k\) is Coulomb’s constant, \(q_{1}\) and \(q_{2}\) are the two different charges exerting a force on each other, and \(d\) is the distance between the centers of the two charges. Note that the sign for each of the charges will impact whether the force between them is attractive or repulsive.
Specifically, if the calculated force is negative (opposite signs on the charges), then the force is attractive. If the calculated force is positive (same signs on the charges), then the force is repulsive.
Note that since distance is in the denominator of the equation, the closer the two charged objects are to each other, the stronger the force is between them.
Electric Fields
Another interesting property of electrically charged objects is the electric field surrounding them. We can think of the electric field as lines emanating from the surface of the charged object. For negatively charged objects, we draw the lines with arrows pointing inward to the object and for positively charged objects, we draw the arrows pointing outward. The closer and denser the lines are, the stronger the field is in that spot.
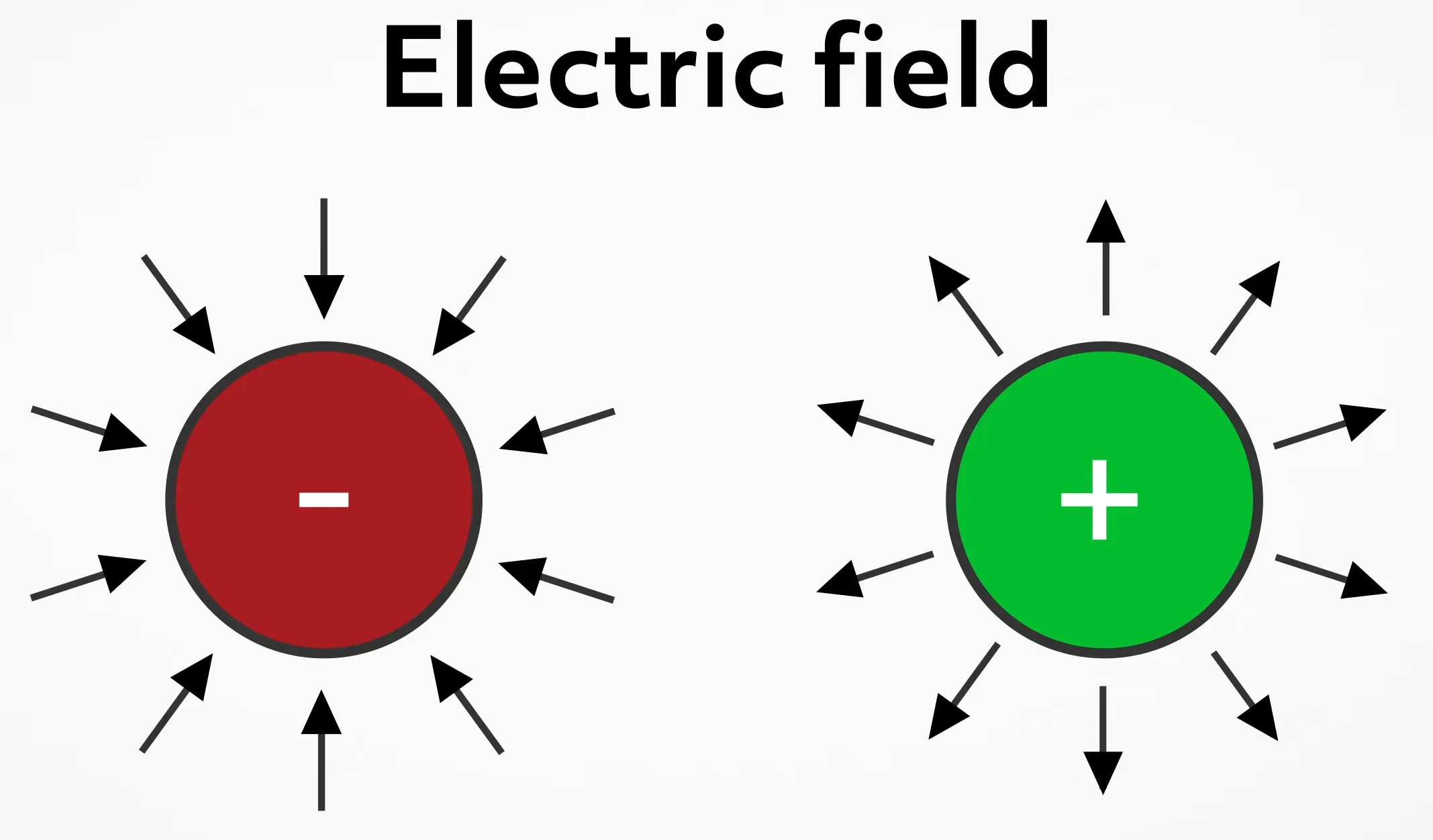
Theoretically, these lines go on forever, but get weaker the further away they are from the charge. For two like charges, electric field lines will avoid each other and the electric force will push the objects apart.
For opposite charges, the electric field lines will actually connect the two charges and the electric force will draw them closer together. Visually, this is demonstrated by lines that extend all the way from the positive charge to the negative charge, indicating an attraction. All charged particles or objects in an electric field will experience the electric force.
The equation for electric field is:
The letter \(k\) is Coulomb’s constant, \(q\) is the charge of the object whose electric field we are measuring, and \(d\) is the distance from the charged object whose electric field we are measuring to the spot where we want to determine \(E\). The value of the electric field changes depending on where you are.
This is similar to the electric force and it can be pretty easy to mix up the two equations. So, it’s important to remember that the electric force describes the interaction between two objects and, therefore, includes a \(q_{1}\) and \(q_{2}\) and the electric field describes the field of one object, so it only has one \(q\).
Static Electricity, Conductors, and Insulators
Charge, of course, is not limited to point particles like this. We can have large objects with a net positive or negative charge, as well. Charge that is built up on objects is called static electricity. Objects handle the build up of charge in a few different ways. The two main categories are conductors and insulators.
Conductors have electrons with the ability to move freely on them and, therefore, have the ability to have a flow of electrical charge through them. Since the electrons can move, the charge will disperse as much as possible for the given shape of the conductor. So, when a conductor, a piece of metal, has attracted extra electrons, it will have a net negative charge dispersed over its surface.
An insulator, on the other hand, does not have the ability to have freely-flowing electrons and cannot have a flow of electrical charge like a conductor. They can still build up a static charge, but it will be localized, since it cannot disperse. Common examples of insulators are glass and wood.
Review Questions
Now that we have an understanding of what electric charge is and how it causes electric forces and fields, let’s think about some examples.
1. What is the net charge of an atom composed of 17 protons, 17 neutrons, and 18 electrons?
- -1
- 1
- 0
- -18
2. Imagine a very large metal plate that has a net negative charge. In the middle of the plate, just above it, you place an ionized helium atom containing 2 protons, 2 neutrons, and 1 electron. What will happen to this atom?
- The atom will move directly upward, away from the plate.
- The atom will move directly downward, toward the plate.
- The atom will remain where it is.
- The atom will move to the right.
I hope this review was helpful! Thanks for watching, and happy studying!