
Hi, and welcome to this video on Alkenes!
Bond Types
Alkenes are unsaturated hydrocarbons, where some of the hydrogens have been replaced by a carbon-carbon double bond. Let’s look at a simple example, starting with the alkane butane and converting it to the alkene, 2-butene.
In butane, the 4-carbon frame is saturated with hydrogens. In 2-butene, two of the hydrogens have been replaced by a carbon-carbon pi bond.
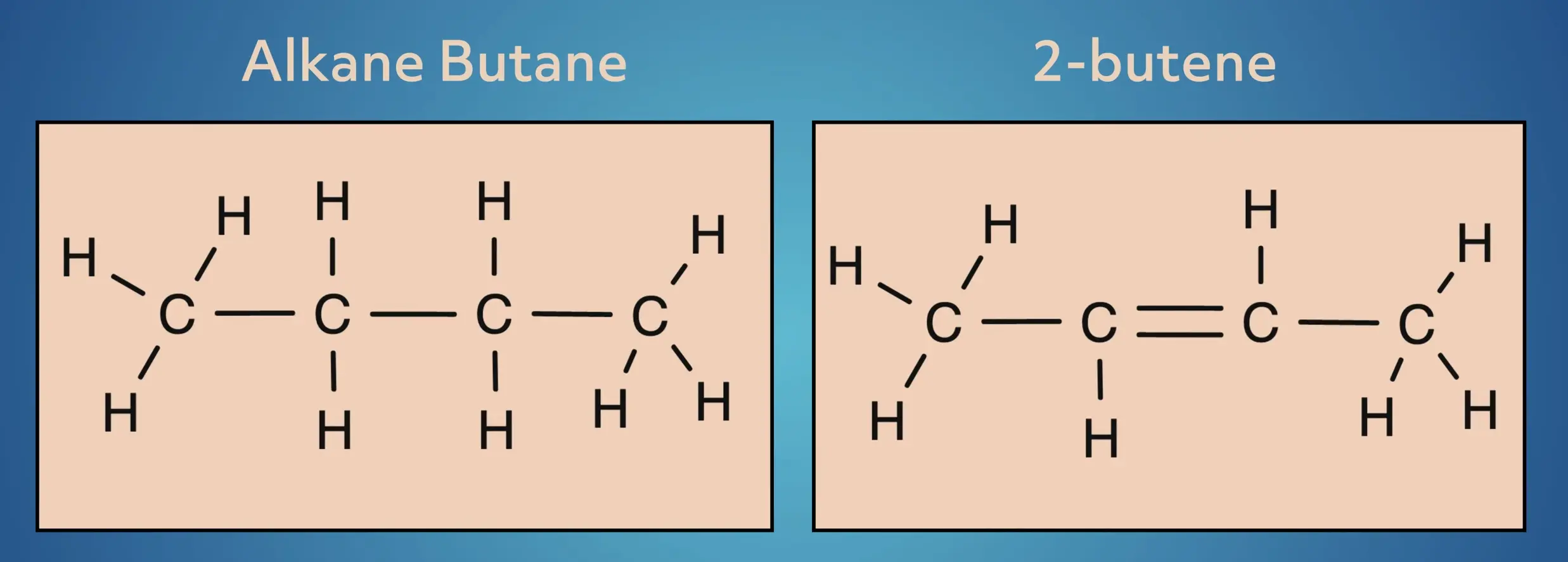
While this may seem like a small adjustment, this greatly changes the structure and reactivity of the molecules.
Let’s consider the structural implications first. The two carbons involved in the double bond are now sp2–hybridized, compared to the terminal carbons, which are sp3–hybridized. This means that the carbons in the double bond have trigonal planar geometry versus the tetrahedral geometry of the sp3–hybridized carbons.
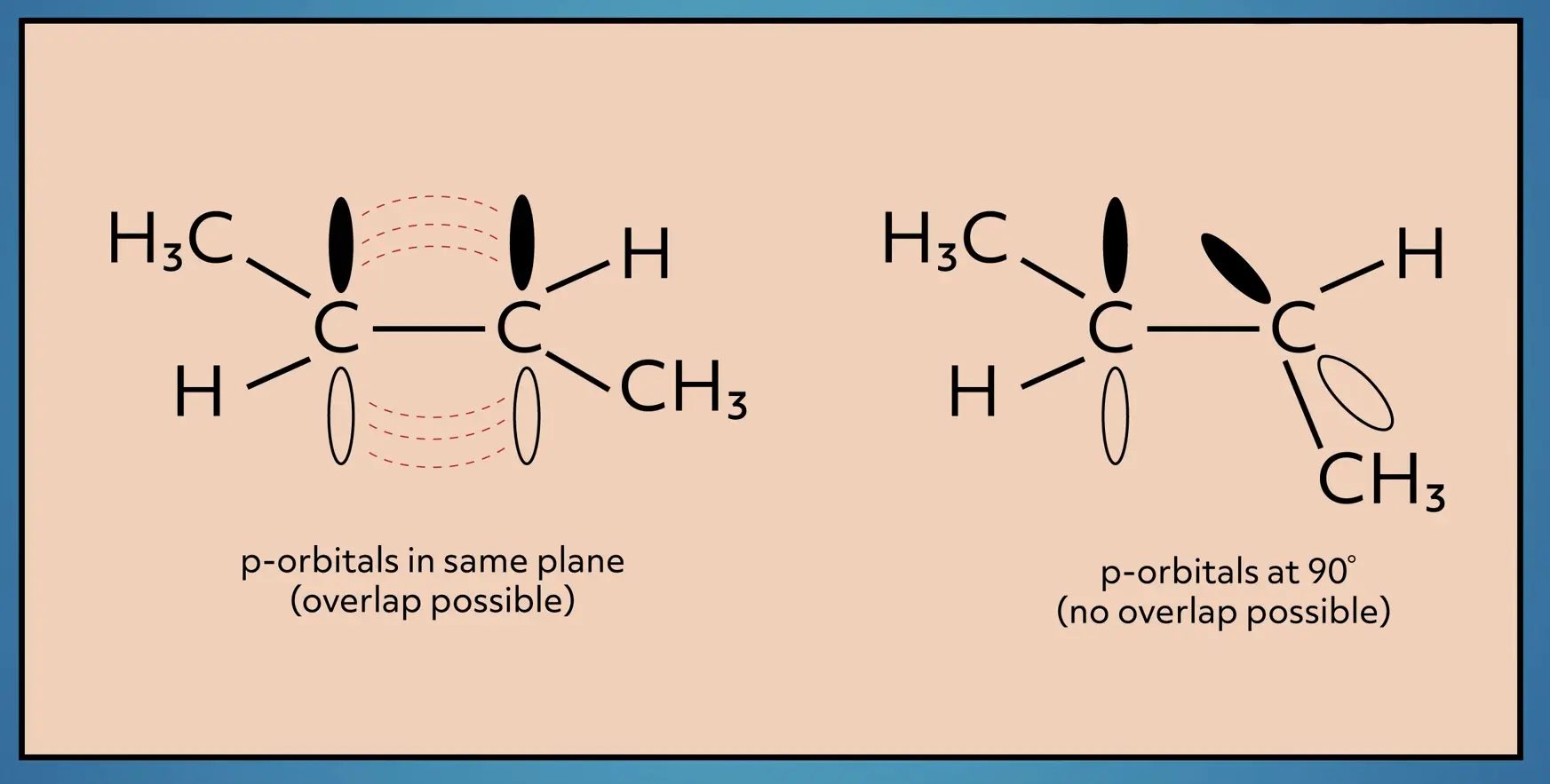
The double bond, which is a sigma bond plus a pi bond, is stronger and shorter than the single sigma bond. It also restricts the rotation of the molecule. This makes a lot more sense when you look at the nature of the pi bond.
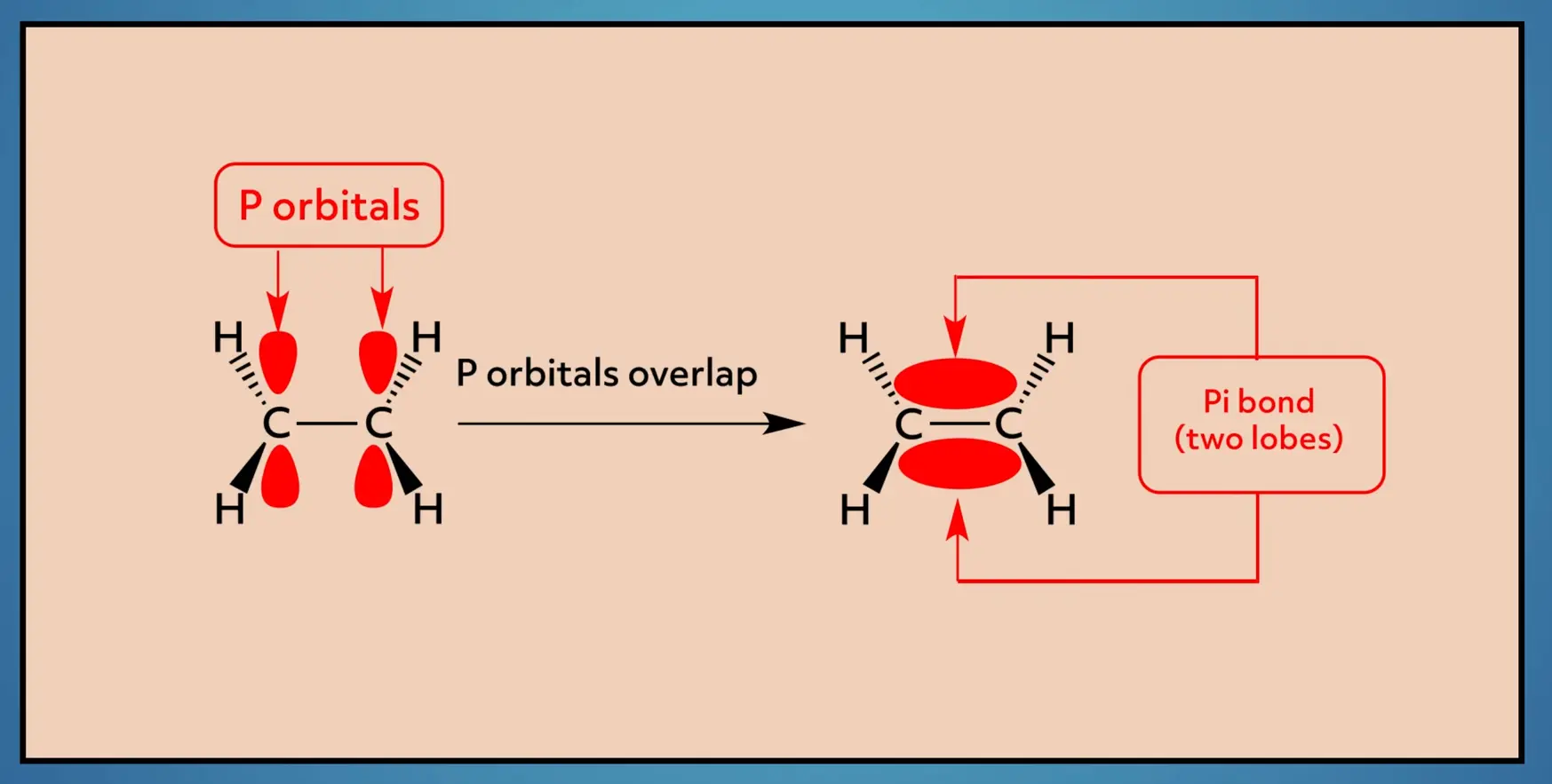
The pi bond is made from the overlap of p-orbitals on each carbon. To maintain the bond, the orbitals need to be oriented in the same plane. This restricts the rotation because as you rotate, the p-orbitals no longer overlap, thus breaking the pi bond, which is, of course, energetically unfavorable.
Structures and Uses of Alkenes
Consider 2-butene again. We can draw the structure in two different ways. One where the methyl groups (CH3) are on the same side of the double bond and one where they are on opposite sides of the double bond. To convert between the two structural orientations, energy is required to break the pi bond. So even though these two structures have the same connectivity, these structures are unique and have different physical properties, because the atoms are locked into different spatial orientations.
We differentiate between these two structures with the names “cis” and “trans”. Cis means the larger groups are on the same side of the double bond and trans means the larger groups are on the opposite side of the double bond.
Remember, cis and trans isomers are only possible in alkenes because of the restricted rotation. Since there is free rotation around the bonds in alkanes, cis and trans isomers do not exist for alkanes.
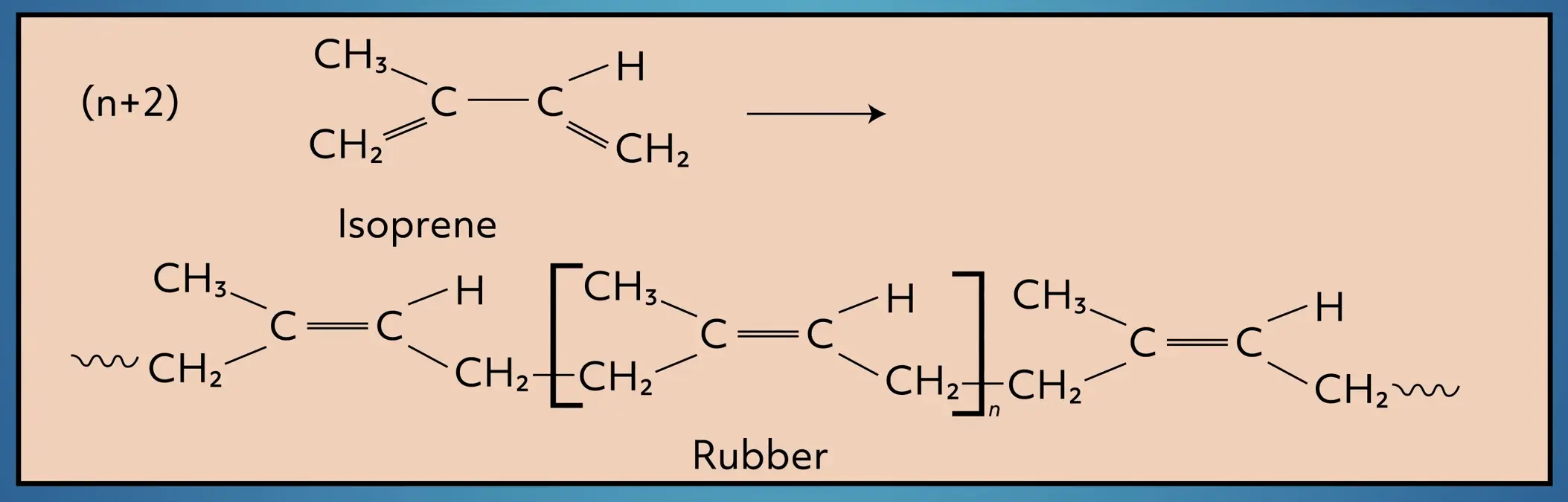
Alkenes can be of varying length, straight-chained or branched, and have multiple double bonds. Take isoprene as an example. Isoprene is a branched diene (two double bonds) that is commonly found in plants and animals.
There are also cyclic alkenes that include carbon rings with double bonds. While according to IUPAC these are technically separate from acyclic alkenes, it’s common to refer to cycloalkenes as alkenes. Beta-carotene, a red-orange pigment in plants and fruits, is an example of a large, cycloalkene.
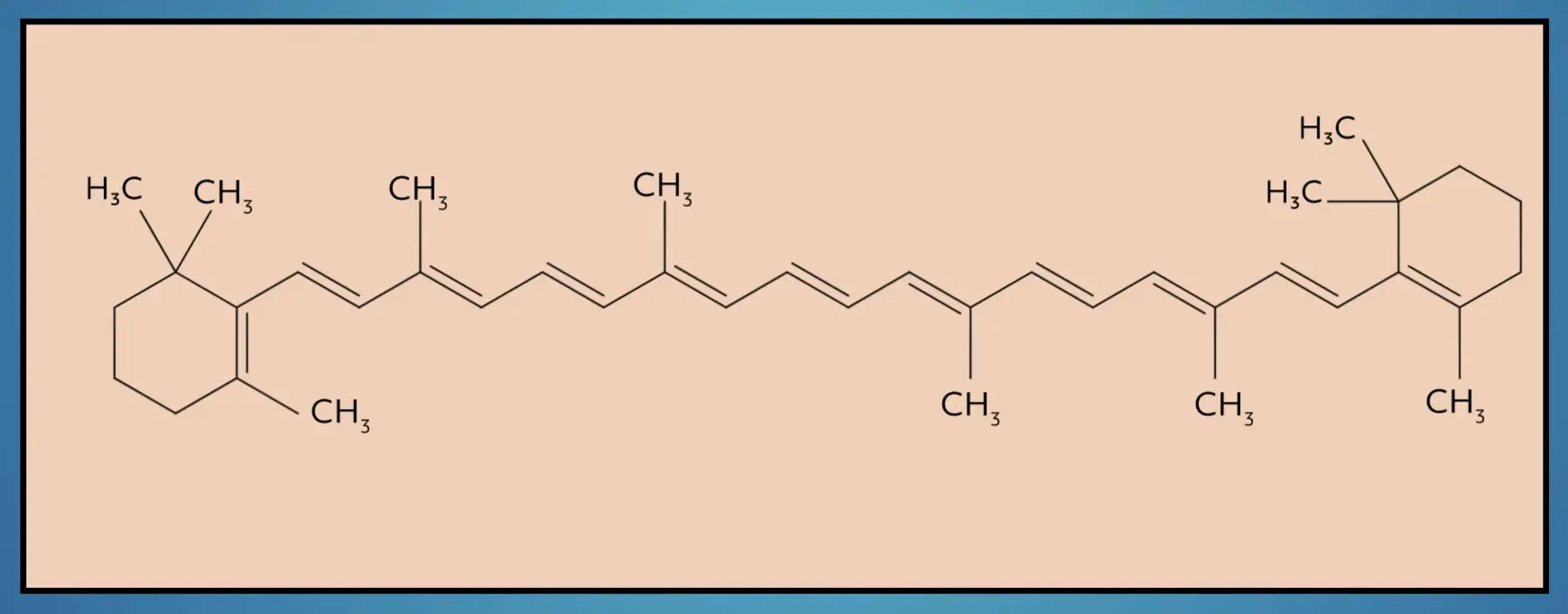
Importantly, while by definition an alkene is an unsaturated hydrocarbon with a double bond, it is common to describe the carbon double bond itself as an alkene.
For example, in the hormone progesterone, while the entire molecule is obviously more complex than a simple alkene, you might hear someone say, “there’s an alkene in the bottom left ring”.
Now that we’ve considered the structure of alkenes, let’s consider their properties. Because alkenes, like alkanes, are hydrocarbons, they are nonpolar and insoluble in water. However, alkenes are generally more reactive than alkanes and are useful starting materials in organic synthesis as functional groups can be added to the carbon frame by replacing the double bond.
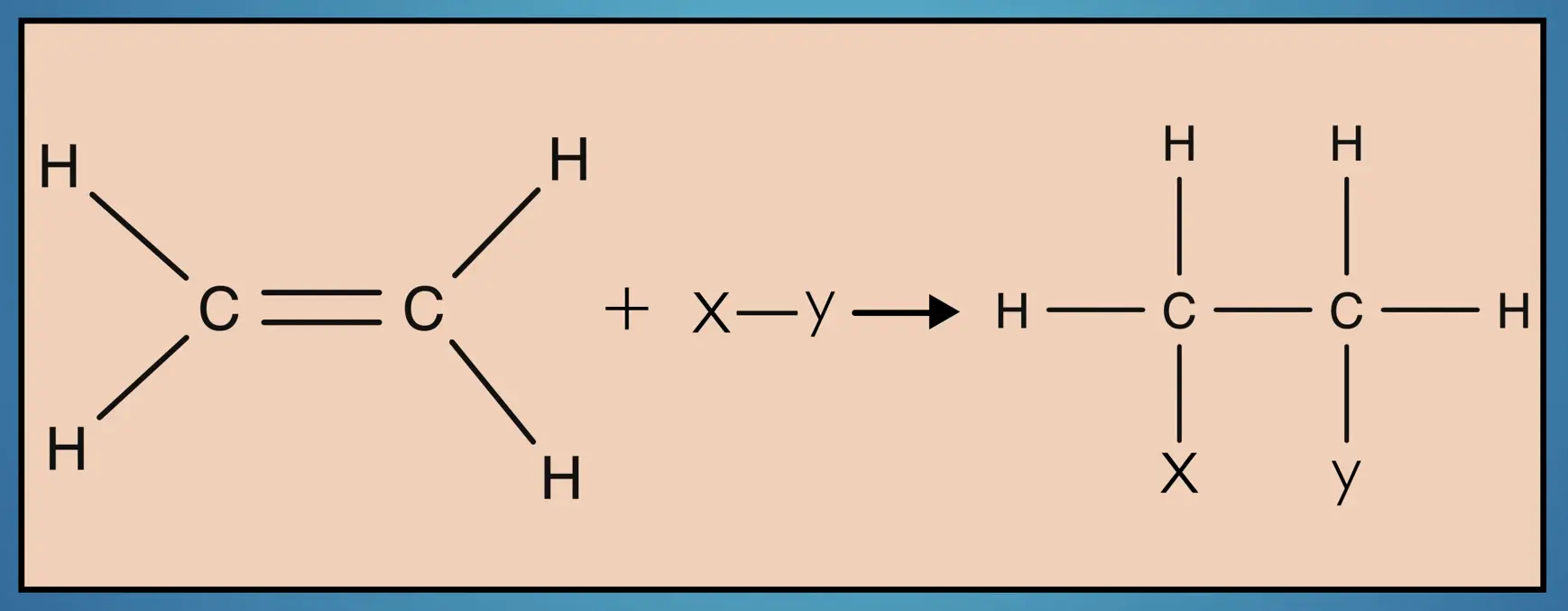
Alkenes are also commonly used in polymerization reactions. For example, isoprene is polymerized to form polyisoprene, a major ingredient in natural rubber.
Review
Let’s quickly review what we’ve covered today.
We first defined an alkene as an unsaturated hydrocarbon with at least one double bond. We then closely inspected the geometry of the double bond, learning that the carbon-carbon pi bond restricts rotation. This leads to cis and trans isomers. We looked at a few examples, showing the great structural variability in the alkene family. And finally, we considered the properties and uses of alkenes in organic synthesis.
Thanks for watching, and happy studying!
