
Hi, and welcome to this video on the states of matter!
A state of matter is a distinct form in which matter can exist. Today, we’ll be looking at the four states that are observable in everyday life: solid, liquid, gas, and plasma.
Solid State
Let’s start with solids. The particles in solids are tightly packed, usually in a specific geometry that repeats itself, called a crystalline structure (there are also amorphous solids where the particles are not in a replicated geometry).
There are four types of crystalline solids, all of which are held together by different forces:
Ionic Solids
Ionic solids, like table salt, which is sodium chloride, are held together by electrostatic attractions
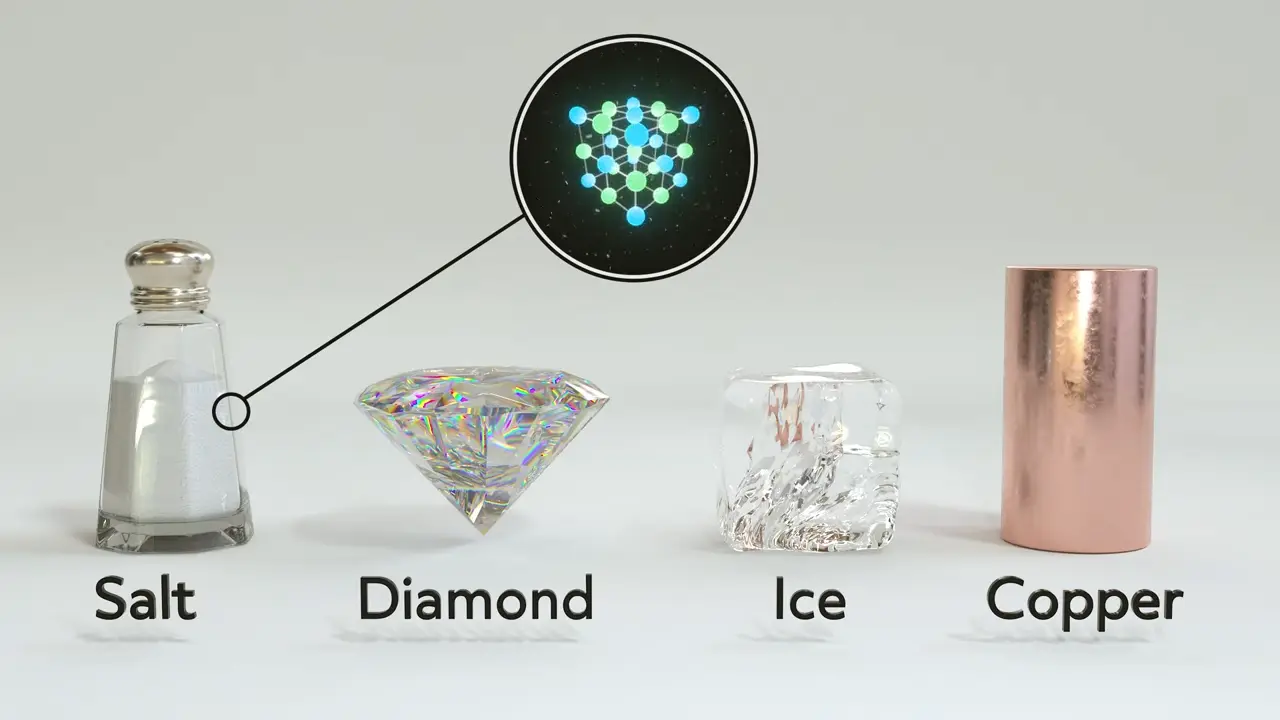
Covalent-Network Solids
In covalent-network solids, like graphite or diamonds, the atoms are connected through covalent bonds

Molecular Solids
Molecular solids, like ice, are held together by many intermolecular interactions.
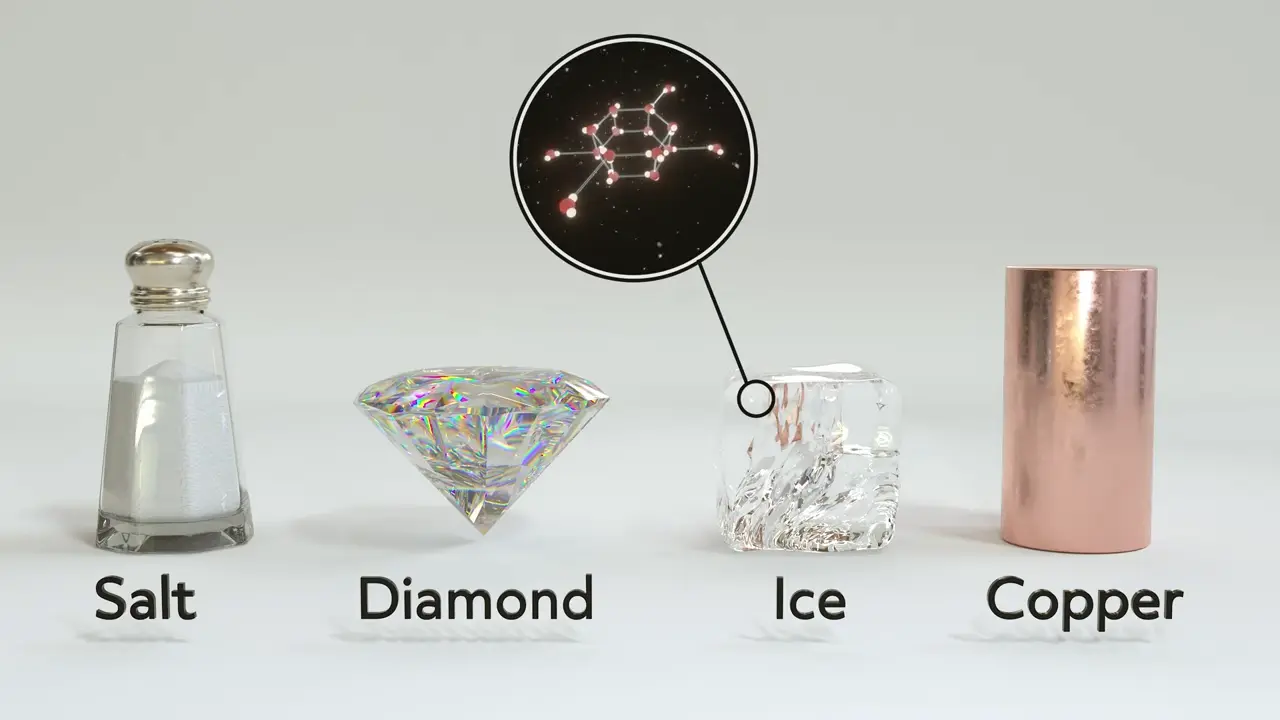
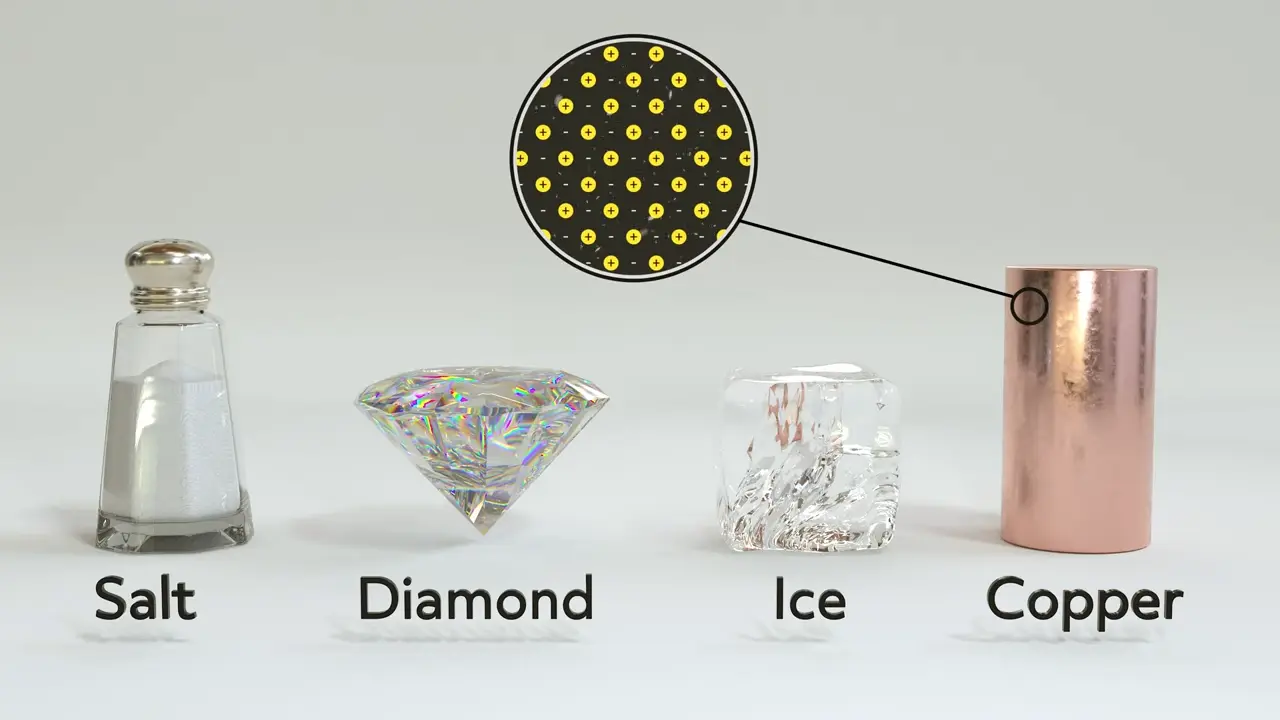
Metal Solids
Metal solids, like copper, are held together by metallic bonds or what is colloquially known as a “sea of electrons.”
As you know from the examples, solids can have very different physical properties (all of which relate back to how the individual particles are connected). However, as solids, they share some physical properties. Solids are usually the densest of the four phases (water is a notable exception) and are therefore nearly impossible to compress. The particles are locked into position and have no translational kinetic energy (however, the atoms or molecules can still be vibrating and rotating in place). Solids hold their shape, rather than conforming to their vessels.
Liquid State
Liquids are also a condensed phase, however, in contrast to solids, the particles have some translational kinetic energy, which means they have freedom to move. The particles still hold onto each other, but have enough energy to continually break away from each other and form new interactions with adjacent particles. This makes liquids flexible, allowing them to flow and take on the shape of the vessel. While liquids are usually less dense than solids, they are still packed tightly enough that they cannot be compressed- in other words, they have a definite volume.

Gas State
Substances transition to the gas phase when the particles have enough energy to completely overcome the interactions holding them together. Consequently, gas particles have lots of translational kinetic energy and are constantly zooming around, vibrating, and rotating. They expand to fill whatever container they’re in and the many tiny bombardments from the particles exert pressure on the container wall. Because there’s so much space between particles, in contrast to solids and liquids, gases can be easily compressed.

Plasma State
Finally, plasma is a highly energetic gaseous mixture of charged particles (though overall, the plasma is neutral). It is considered separate from the gas phase because it has unique physical properties. Like gas, plasma expands to fill its container and is compressible, but very different from standard gases, plasma has high electrical conductivity. This is because some of the charged particles moving around freely are electrons. While naturally-occurring plasma is rare on Earth, our stars are made of plasma, making it the most common phase in the universe.
Now that we’ve covered each phase of matter separately, let’s consider them together.
You’ll notice that as we progressed from solid to liquid to gas to plasma, the internal energy of the system increased. Consequently, it’s often possible to prompt a phase change by simply increasing the temperature of the system. By providing thermal energy, we can break the forces holding particles together and increase the translational kinetic energy of the particles, which typically produces a phase change.
The amount of heat necessary to undergo a phase change depends on the type of material. Returning to the four solids we discussed, usually the forces holding ionic, covalent-network, and metal solids together are much stronger than the forces in molecular solids and consequently have much higher melting points. For example, sodium chloride and copper melt at 1,474 ºF and 1,984ºF, respectively, versus ice, which melts at 32ºF.
In addition to temperature changes, you can prompt a phase transition by adjusting the pressure. The way a substance changes with pressure and temperature is often mapped out in a phase diagram. While these are material-specific, in general, substances tend to be solids at low temperatures and high pressures, gaseous at high temperatures and low pressures, and liquid at intermediate temperatures and pressures.
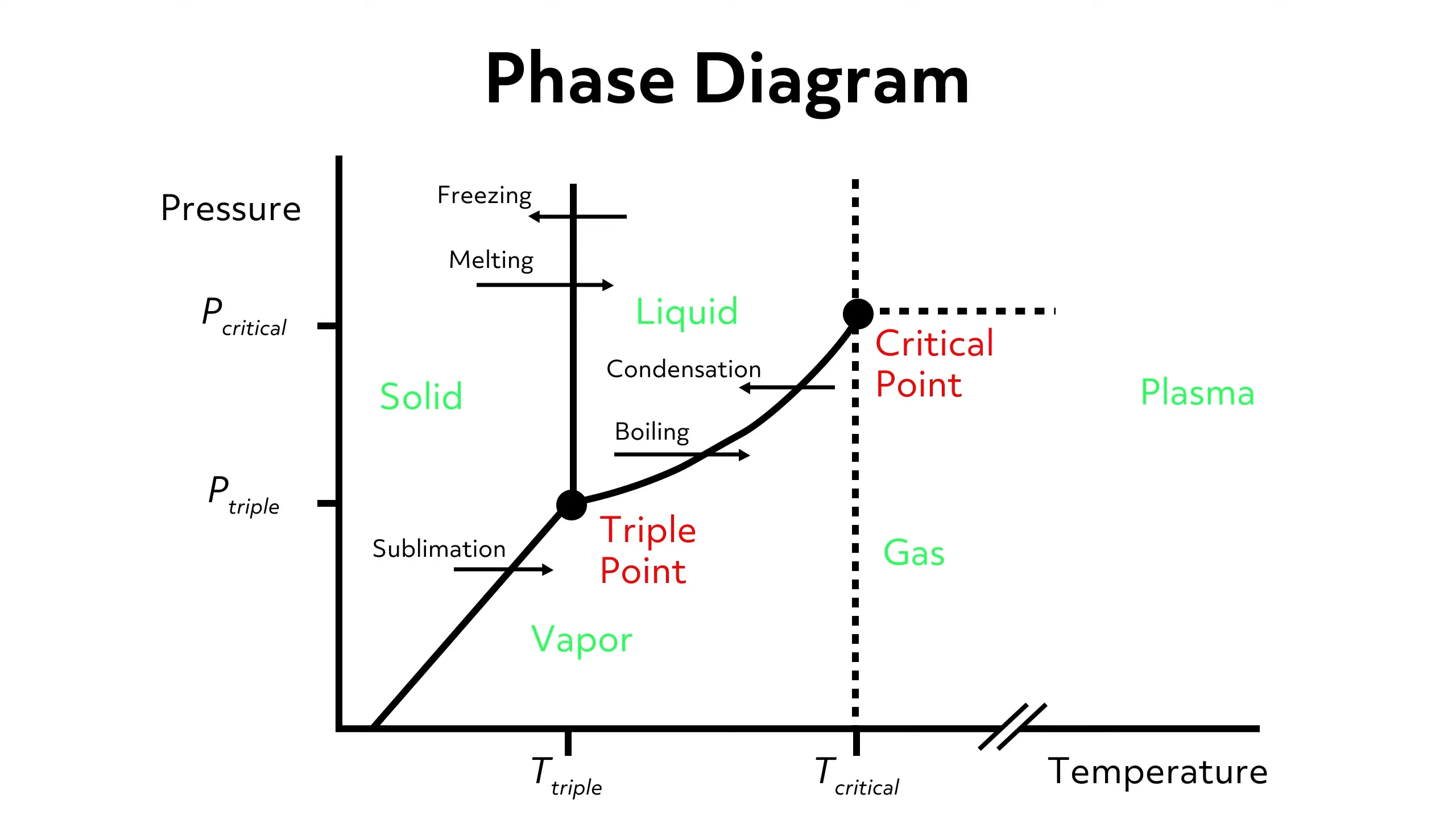
Since most of us encounter only standard pressure and temperatures, we often think of materials as always being in a specific state of matter (for example, most of us think of salts and metals as being solids). But as you can tell from the graph, it’s possible to convert between phases; it just might require extreme temperatures and pressures.
Let’s finish with a quick review. We defined the four states of matter and connected their atomic structure to their bulk physical properties. We noted that the internal energy increases when transitioning from solid to liquid to gas to plasma and discussed how the phase of a material changes with temperature and pressure.
Thanks for watching, and happy studying!