
Alkanes, alkenes, and alkynes are three of the most important and ubiquitous types of hydrocarbon molecules. Hydrocarbons are organic compounds consisting only of carbon and hydrogen atoms. Alkanes such as methane and propane are important due to their combustibility. Alkenes and alkynes are highly reactive and are used to synthesize many important substances, such as alcohols, antifreeze, detergents, polyethylene, vinyl plastics, and acrylics.
The alk- root in the names alkane, alkene, and alkyne identifies them as aliphatic compounds, meaning they consist of carbon chains that do not contain aromatic rings. Alkanes are called saturated hydrocarbons because they have only single covalent bonds between carbon atoms. Both alkenes and alkynes are unsaturated hydrocarbons because they contain at least one double or triple carbon-carbon bond (alkenes have double bonds, and alkynes have triple bonds).
Because of the large number of possible molecular variations in alkanes, alkenes, and alkynes, these groups contain thousands of individual compounds. In the early days of organic chemistry, the naming of the ever-increasing number of organic compounds was not systematic. Compounds were named based on trivial information such as the natural materials from which they were synthesized.
As the number of compounds increased, this ad hoc tradition of naming compounds was replaced with a set of rules for nomenclature developed by the International Union of Pure and Applied Chemistry (IUPAC) to make the study of hydrocarbon groups more efficient.
Alkane Nomenclature
IUPAC nomenclature is best understood by starting with how alkanes are named and then adjusting these names to specify more complex compounds. Three of the rules for IUPAC nomenclature of alkanes are as follows:
Rule #1
First, identify the longest continuous carbon chain (also called the parent chain) within the molecular structure and apply the following convention: if the chain is two carbons long, it is called ethane; if it is three carbons long, it is propane; and if it is four carbons long it is called butane. Longer chains such as the five-, six-, seven-, eight-, nine-, and ten-carbon chains are named pentane, hexane, heptane, octane, nonane, and decane respectively.
Rule #2
Second, add prefixes to identify its substituents. A substituent is a group of atoms that branch off of the parent carbon chain. The position of a substituent is determined by a numerical value corresponding to its location on the parent chain. Positions along the chain are numbered by consecutively counting carbon atoms from either end of the molecular formula. IUPAC naming rules determine how the carbon atoms of the parent chain are numbered.
Rule #5
Third, if there are multiple substituent groups present, they are listed alphabetically. And if there are multiple substituents of the same type, they are denoted using the prefixes di-, tri-, tetra-, etc.
Another important aspect of this rule is that, by convention, the carbon atoms along the parent chain are numbered according to the location of substituents. The carbon numbering begins at the end of the chain closest to a substituent group. Therefore, the number of carbon atoms may begin on the left side of the molecular formula or the right side, depending on the substituents’ positions. This convention aims to assure that the substituent groups receive the lowest possible number along the carbon chain formula.
Alkane Nomenclature Example
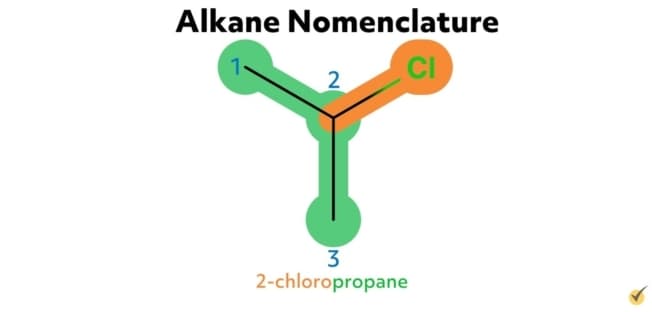
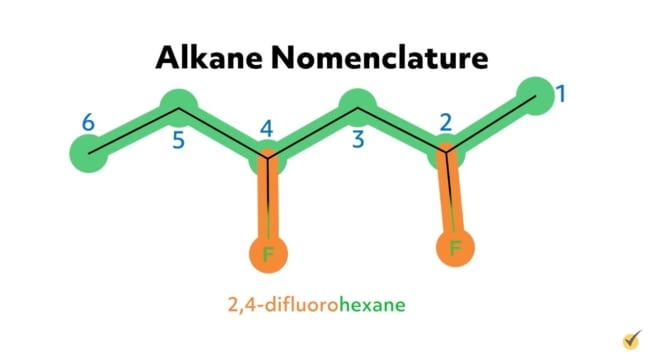
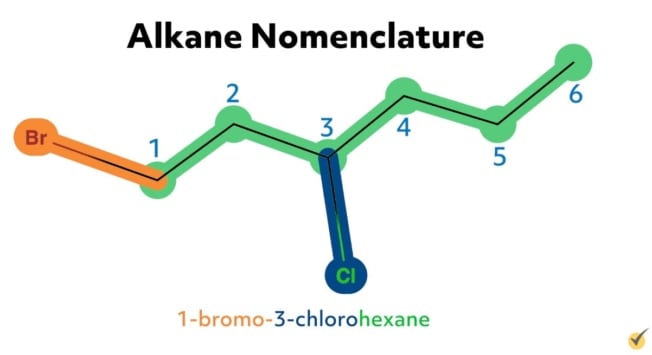
For example, the alkane 2-chloropropane is one in which there are three carbon atoms in the chain (thus propane) with a chlorine substituent at the second carbon on the parent chain. Similarly, 2,4-difluorohexane is a compound with six carbons in its chain (hexane) with two fluorine substituents (difluoro) at the second and the fourth positions along the parent chain. 1-bromo-3-chlorohexane has a bromide substituent at the first position and a chlorine substituent at the third position along the hexane chain.
Alkene Nomenclature
The rules for naming alkenes are similar to those of alkanes. However, the -ane ending is replaced with -ene to indicate that these compounds have at least one carbon-carbon double bond. The number of carbon atoms in the parent chain of– alkenes follows the same naming convention used for alkanes.
So ethene has two carbon atoms, propene has three, and so on for butene, pentene, hexene, heptene, octene, nonene, and decene. The rules specific to alkenes are that:
Rule #1
First, the longest continuous carbon chain containing both carbon atoms of the double bond is identified (this is the parent chain).
Rule #2
Second, the numbering of carbon positions along the chain is ordered to give the lowest possible number for the doubly bonded carbon atoms. If the double bond is equidistant from both ends of the chain, then the carbon atoms are numbered to minimize the numbers assigned to substituent groups.
Rule #3
Third, the position of the double bond is placed directly before the name of the parent chain. The substituent groups are added as prefixes in alphabetical order. A suffix, such as -diene, -triene, etc., is used if multiple sets of double bonds exist.
Alkene Nomenclature Example
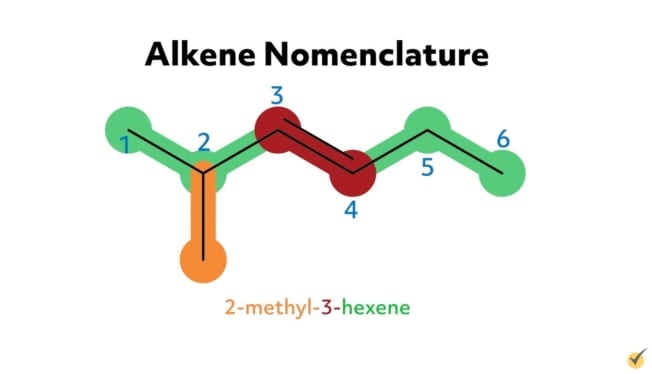
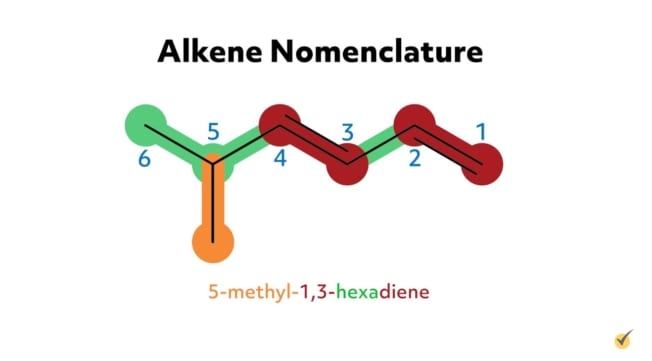
For example, 2-methyl-3-hexene has six carbon atoms in its parent chain, a methyl substituent group at the second position, and a double bond at the third position on the parent chain. 5-methyl-1,3-hexadiene has six carbon atoms in its parent chain, a methyl substituent at the fifth position, and two double bonds, one at the first position and another at the third carbon position. The -diene suffix indicates that there are two carbon double bonds.
An IUPAC recommendation in the early 1990s indicates that the location number of the double bond in an alkene can come before the suffix (i.e., before “-ene,” “-diene,” “-triene,” etc.). For example, 5-methyl-1,3-hexadiene could also be written as 5-methylhexa-1,3-diene. The goal is to be as clear as possible in the naming.
Alkyne Nomenclature
Alkynes are defined by the presence of at least one carbon-carbon triple bond and are denoted by the suffix -yne. Following the convention used for alkanes and alkenes, the number of carbon atoms in the parent chain of alkynes (1–10) are indicated as ethyne, propyne, butyne, pentyne, hexyne, heptyne, octyne, nonyne, and decyne. The IUPAC rules that are specific to the alkynes are as follows:
Rule #1
First, the parent chain, containing both carbons of the triple bond, is identified.
Rule #2
Second, the numbering of carbon positions along the chain is ordered to give the lowest possible number for the carbon-carbon triple bond. If the triple bond is equidistant from both ends of the chain, then the carbon atoms are numbered to minimize the numbers assigned to substituent groups.
Rule #3
Third, the carbon number of the triple bond is placed directly before the parent chain name. The substituent groups are added as prefixes in alphabetical order and according to their position. If there are multiple occurrences of the same substituent, the prefixes di-, tri-, and tetra- are used. The suffix -diyne is added to the compound name to indicate the presence of two triple bonds.
Rule #4
Fourth, if an alkyne contains both a double bond and a triple bond, the parent chain is numbered so that the multiple bond closest to one of the ends of the chain gets the lowest number. If triple and double bonds are the same distance from different ends of the chain, the carbons are numbered such that the location of the double bond receives the lowest value.
In cases with both triple and double bonds, the molecule is named “n-en-n-yne,” with the double bond root name preceding the triple bond root name.
It should also be noted that when substituents contain carbon-carbon triple bonds they are named alkynyl. So, names containing ethynyl, propenyl, or butenyl, for example, indicate compounds with substituents containing triple bonds.
Alkyne Nomenclature Example
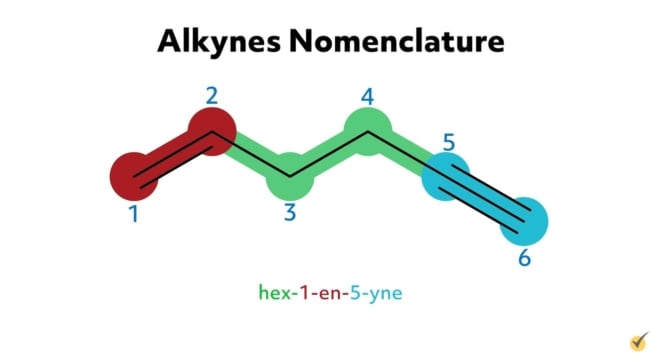
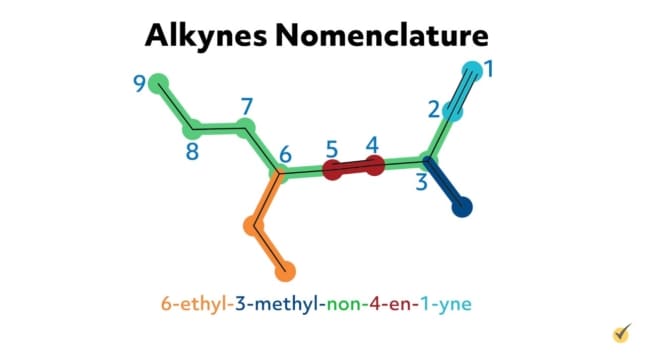
For example, hex-1-en-5-yne is an alkyne with six carbon atoms in its parent chain, a double bond at the first carbon position on the chain, a triple bond at the fifth carbon position, and no substituent groups. 6-ethyl-3-methyl-non-4-en-1-yne is an alkyne with nine carbons in its parent chain, an ethyl substituent group at the sixth carbon position, a methyl substituent at the third carbon position, a double bond at the fourth position, and a triple bond at the first position on the chain.
IUPAC nomenclature for alkanes, alkenes, and alkynes provides an international standard for naming these important and ubiquitous hydrocarbon compounds. These systematic naming rules allow a chemist to know the type of compound, the length of the parent carbon chain, and the molecular position of substituent groups, double bonds, and triple bonds. IUPAC nomenclature for these hydrocarbons thus captures many of the main principles of organic chemistry.
Let’s go over a few review questions before we go:
Review Questions
1. Identify the following compound as an alkane, alkene, or alkyne and indicate how many carbon atoms are in its parent hydrocarbon chain.
It is an alkane, as indicated by the -ane suffix, and has seven carbon atoms in its parent chain, as indicated by the hept- prefix.
2. Identify the following compound as an alkane, alkene, or alkyne. How many carbon atoms are present in its parent chain? Does this molecule contain any double or triple carbon-carbon bonds? If so, indicate how many and their positions along the parent carbon chain.
This is an alkyne as indicated by the -yne suffix. It has eight carbon atoms in its parent chain (as shown by “octa”) and two triple bonds at the second and fourth positions (as indicated by “2,4-diyne”).
That’s all we have for now! Thanks for watching, and happy studying.
