
Hi, and welcome to this video on acids and bases!
Historical Classification
Let’s get started with a little history, a deep dive into how acids and bases were first classified.
Back in the 17th century, before scientists knew anything about the molecular structure of acids and bases, they gave them definitions based solely on their observable properties. So, an acid was any substance that
- Tasted sour
- Reacted with metals to produce hydrogen gas
- Changed the color of certain organic compounds (now known as indicators)
On the other hand, bases were any substance that
- Tasted bitter
- Felt slippery
- Turned those same organic compounds a different color
Chemists began exploring acid-base chemistry and further added to their definition that when mixed together, acids and bases were neutralized, canceling out each other’s properties.
While these early definitions still apply to many acids and bases, we now have the Brønsted-Lowry acid-base definition, a broader description based on their molecular structure.
Brønsted-Lowry defines an acid as any species that donates a proton, and a base as any species that accepts a proton. So now, following this definition, instead of considering just the observable properties, chemists can assess the strength of acids and bases on how likely they were to give or receive a proton. This approach does a much better job of explaining the phenomena of all acids and bases.
Acid Dissociation and Strength
So let’s delve into this a bit more, starting with acids.
When a generic acid is added to water, the acid dissociates, releasing the proton to water to form the conjugate base of the acid and the hydronium ion.
As you’ll notice by the double arrow, this is an equilibrium process. The strength of the acid is determined by how much of the acid actually dissociates when added to water. Strong acids completely dissociate when added, whereas only a portion of a weak acid dissociates, which means some generic acid always remains in solution.
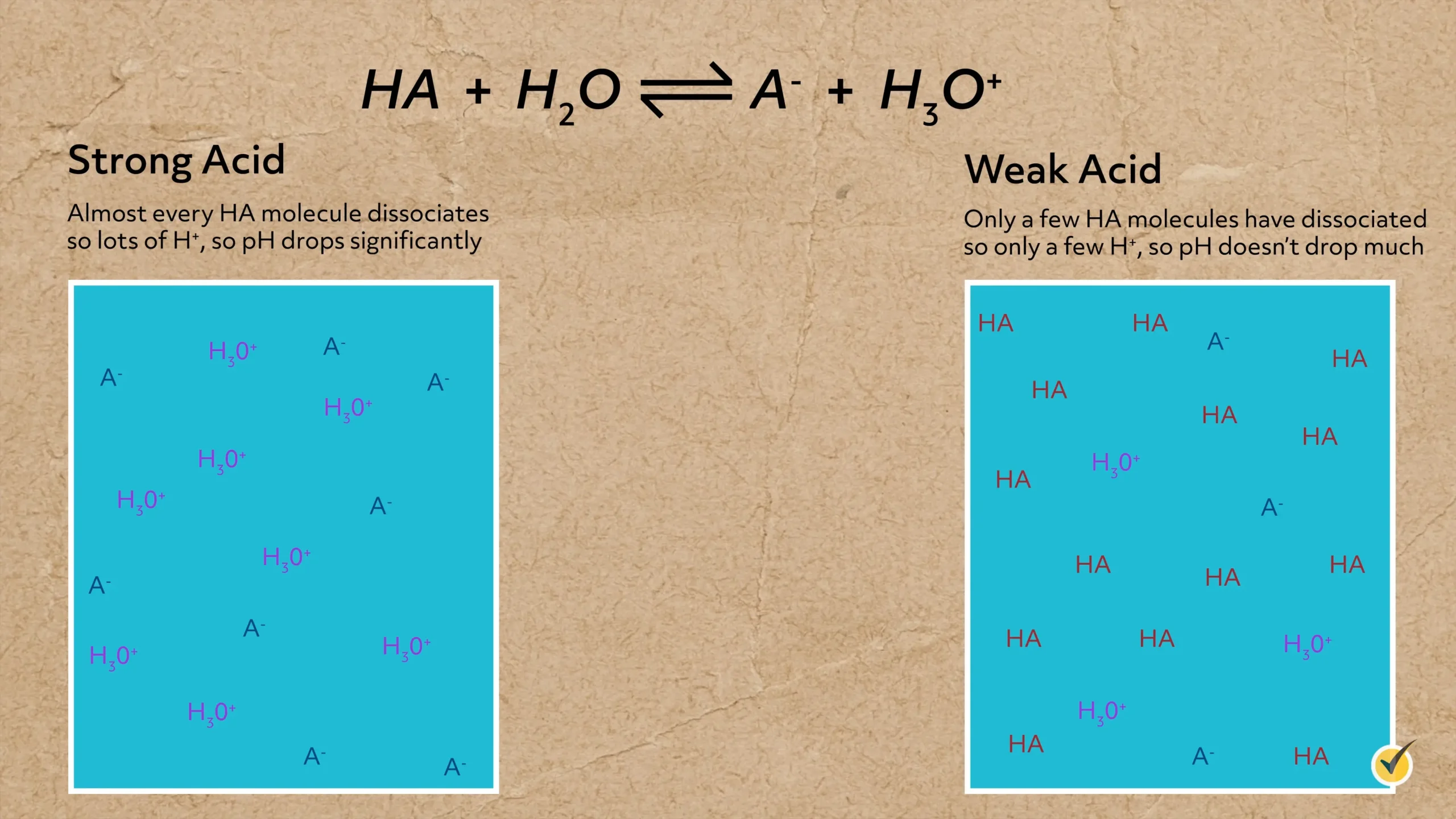
The strength of an acid can also be assessed by the stability of its conjugate base. When the conjugate base does a good job of stabilizing the negative charge (which means it has a lower energy), more acid will dissociate.
For example, hydrochloric acid is a strong acid and completely dissociates into hydronium and chloride because chloride has a high electron affinity and gladly accepts the additional electron.
In other words, it does a good job stabilizing the negative charge.
In contrast, acetic acid is a weak acid and only partially dissociates when added to water because the conjugate base is not quite as capable of stabilizing a negative charge.
We can quantitatively describe the dissociation of acids using the acid-dissociation constant, \(K_{a}\) (which is just an equilibrium constant). As the strength of the acid increases, the concentrations of hydronium ions and the conjugate base increase, which increases our dissociation constant..
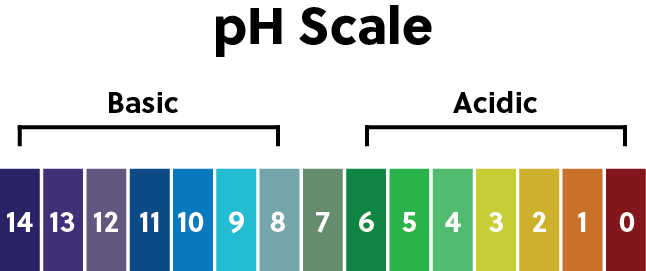
We typically monitor the pH of a solution to measure the concentration of hydronium ions. As the concentration of \(H_{3}O^{+}\) increases, the pH decreases.
Any pH below 7 is considered an acidic solution, and any pH above 7 is basic. And 7 itself, which is the pH of pure water, is neutral.
A similar approach is used to understand bases, except now we consider the stability of the conjugate acid, \(\text{HB}+\), in the base reaction with water.
Again, strong bases completely react, while weak bases only partially deprotonate water.
For example, ammonia is a weak base, and in a 0.1 molar solution, only approximately 1% reacts with water.
The strength of the base increases with the stability of the conjugate acid. For example, exchange two of these ammonia hydrogens for ethyl groups to make diethylamine, and now, in the same 0.1 molar solution, about 9% of the base reacts with the water.
This primarily happens because the two ethyl groups contribute electron density to the nitrogen, making it better suited to stabilize a positive charge.
However, diethylamine is still a weak base. Much stronger bases are anions from salts like sodium hydroxide, which, of course, do a great job of stabilizing a proton, because they react to form water.
Just like \(K_{a}\), the strength of a base can also be quantified by an equilibrium constant, in this case, the base-dissociation constant, \(K_{b}\).
Though not used for everyday reactions, there are even stronger acids and bases, known as superacids and superbases. These compounds are highly reactive because one is extremely motivated to release a proton and the other to receive a proton. Basically, it’s very energetically favorable for them to undergo the reaction.
Triflic acid, short for trifluoromethanesulfonic acid, is an example of a superacid. It substitutes trifluoromethane for one of the oxygens and the second hydrogen on sulfuric acid, which helps to stabilize the conjugate base, making it an even stronger acid than sulfuric.
Lithium diisopropylamide, LDA, is an example of a superbase. The lone pair on the nitrogen is highly basic and is useful in organic synthesis when very weak acids need to be deprotonated for the reaction mechanism.
Now that we have a molecular understanding of acids and bases, let’s return to some of the observable properties that 17th-century scientists identified and try to understand them at a deeper level.
Of course, we now know that when acids and bases neutralize each other, it’s because the acid hands off a proton to the base, thus immediately completing both the acid and base reactions and leaving the solution with the more stable conjugate acid and conjugate base.
And when acidic and basic solutions alter the color of indicators, like when litmus paper is used to test the pH of a solution, it’s because an organic compound on that litmus paper is either protonated by an acid or deprotonated by a base, and that molecular change translates into a color change.
We can also understand why acidic and basic solutions conduct electricity. Electricity is the movement of charge. When acids and bases react with water, charged species are generated, which then allows for the conductance of electricity. And as you might expect, the conductance increases with the strength of the acid and base because ion concentrations are also increasing.
Another one of the observable properties is the sour taste of an acid. Acids, like citric and acetic, taste sour because the sour taste receptors on our tongues are triggered by the hydronium ions.
Lastly, it is observed that some bases feel slippery. This is because when they react with the macromolecules on your skin (like fatty acids), they produce molecules with the same structure as soap.
So, as you can see, with the Brønsted-Lowry definition, we can actually understand the underlying chemistry that produces the properties observed by 17th-century chemists.
Review
All right, let’s wrap things up with a quick review.
Back in the 17th century, definitions of acids and bases were largely based on their observable properties. Today, we use the Brønsted-Lowry definition, in which acids are compounds that donate protons and bases are compounds that accept protons. These definitions are broad and can be used to explain the difference between strong and weak acids and bases. Finally, the strength of acids and bases can be assessed by the stability of their conjugate base and conjugate acid, respectively.
That’s all for this review! Thanks for watching, and happy studying!