
Hi, and welcome to this video about naming alcohols!
Today, we’re going to cover how to name alcohols using the naming conventions of the International Union of Pure and Applied Chemistry (IUPAC). These names are separate from the common names used in day-to-day life, providing a systematic nomenclature for chemists and giving structural information about the compound.
First, let’s quickly remind ourselves of what an alcohol is chemically. All alcohols contain a hydroxyl group (which is hydrogen bonded to oxygen, represented as OH) bound to carbon.
Alcohol Structures
To name an alcohol, we first need to describe the carbon chain it’s attached to. We’ll start by counting the longest continuous carbon chain in the compound and using the IUPAC name for that alkane as the root word for the compound. Here are the names for alkanes up to 10 carbons long.
Let’s begin with the simplest alcohol, which has the formula HOCH3. Since the longest carbon chain is one, we use methane as our root word. Notice that instead of having a fourth hydrogen attached to the carbon, there is a hydroxyl group, so the name must change to reflect that. We do this by replacing the terminal -e in methane with –ol. So instead of methane, we now have methanol.
Now let’s try it with a three-carbon chain (C3H7OH). Our approach starts off the same. We find the longest carbon chain to be three, so our root word is propane. To indicate the addition of an alcohol, we again replace the terminal –e with –ol, giving us propanol. But now we’re left with an important question: Where on the carbon chain is the alcohol? It could be bonded to either of the terminal carbons or to the middle carbon.
To specify which carbon the alcohol is bonded to, we start by numbering the carbons and then use the number to indicate where the alcohol is attached. Choosing to start from the left carbon, we number them 1, 2, and 3. For the first compound, the alcohol is attached to the second carbon, so it is named propan-2-ol. The 2 tells us that the alcohol is attached to the second carbon.
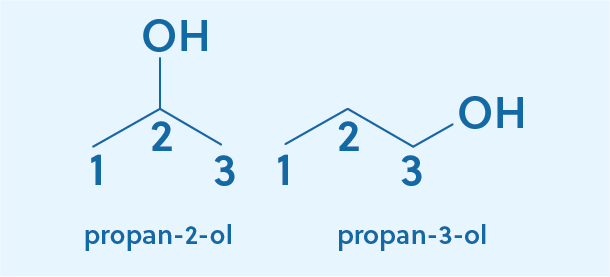
For the second compound, the alcohol is attached to the third carbon, which would lead us to name the compound propan-3-ol. However, that name is actually never used! Notice that if the alcohol was on the first carbon, it would be chemically identical to when it is attached to the third carbon, meaning propan-1-ol and propan-3-ol are the same compounds. In cases like this, the convention is to use the smaller number, so propan-1-ol is the correct name.
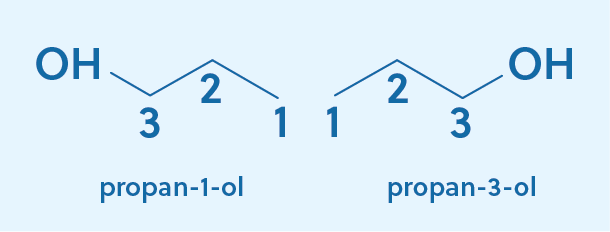
However, while propan-1-ol is the formal IUPAC name, you will often see the 1 dropped and the compound referred to as propanol. If no number is included in the name, you can assume that the alcohol is on the first carbon.
Applying IUPAC Rules
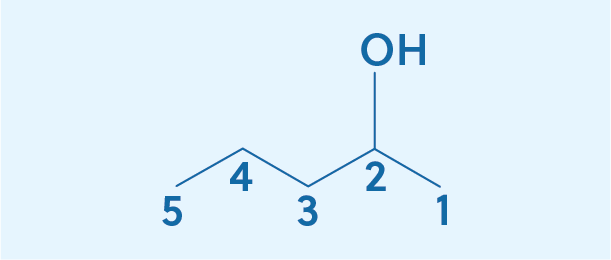
Now let’s try naming this compound:
We’ll start by numbering the carbons. 1-2-3-4-5:
There are five carbons, so the root word is pentane. The alcohol is attached to the second carbon, giving us pentan-2-ol. Note that if we had counted from the left, the alcohol would be attached to the 4th carbon and we could have named this pentan-4-ol. But since 2 is less than 4, pentan-2-ol is the correct name. You want to call it by the lowest number, so start counting from whichever side will give you the lowest number.
While pentan-2-ol is the formal IUPAC name, for such simple compounds, the number is often moved to the front of the word. In this example, that gives the name 2-pentanol. Since there are no other functional groups, the 2 must refer to the position of the alcohol.
Let’s review what we’ve learned about naming simple organic alcohols. First, it is very helpful to know the IUPAC names for alkanes up to length 10 because we’ll use these as our root words. When you start to name a compound, find the longest continuous carbon chain and use that alkane name as your root. Replace the terminal –e with –ol to indicate the presence of an alcohol functional group. Then, include a number between the root word and –ol to indicate where on the carbon chain the alcohol is attached. If the alcohol is on the first position, convention allows us to drop the -1. If there are two identical carbons (like for pentan-2-ol and pentan-4-ol) always use the smaller number.
As you can imagine, naming can get a bit complicated as you encounter larger and more complex molecules. But once you master a few simple rules, naming will be second nature!
Thanks for watching, and happy studying!