
In this video, we will investigate the type of bonding in metals, like copper, and metal alloys, like brass, compare them to covalent and ionic bonds, and discuss a few common types of metals and a few of their characteristics.
What are Metallic Bonds?
Metallic bonds are a unique type of intramolecular bond that, as the name implies, takes place between the atoms in metals and metal alloys.
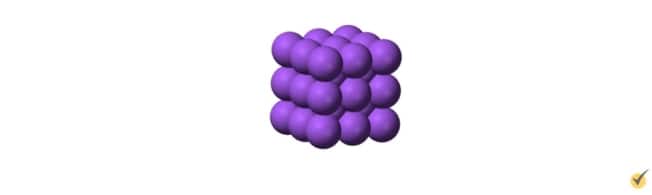
A metal or metal alloy typically has a crystalline structure with a uniform arrangement of atoms throughout the substance.
The primary attribute that differentiates metallic bonds from covalent bonds and ionic bonds is based on the relative electronegativities of the atoms involved. Each of these bond types results in different behaviors for the associated electrons.
Ionic Bonds
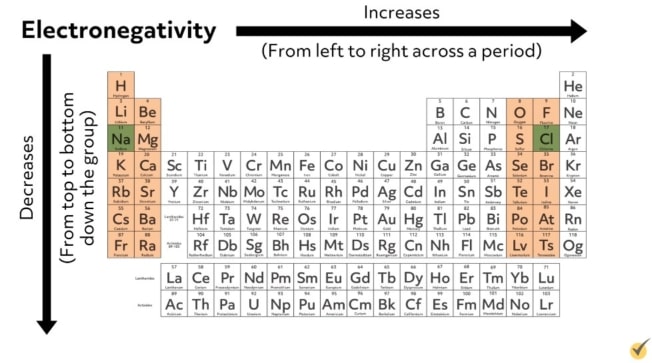
As a reminder, elements on the top right side of the periodic table have high electronegativities, and elements on the bottom left of the periodic table have low electronegativities. Because of this, ionic bonds occur between elements on the right and left sides of the periodic table that have a large difference in electronegativity like in sodium chloride.
Typically, the more-electronegative elements in an ionic bond “steal” the electron from the less-electronegative element, and the result is two oppositely charged ions.
In this example, the chlorine atom is the more electronegative element, and the sodium atom is the less electronegative element.
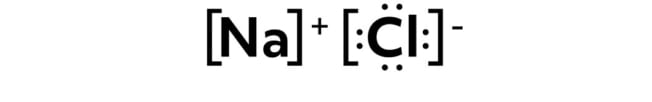
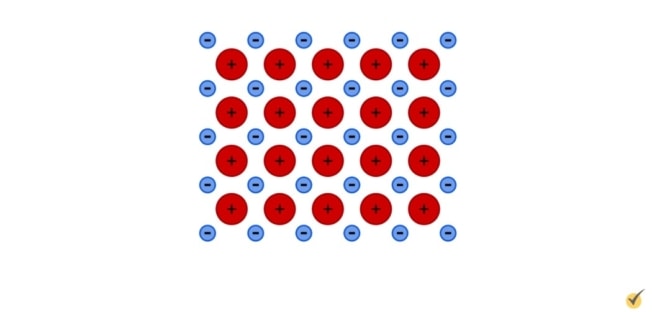
So in ionic bonds, the electron (or electrons in some cases) leaves one atom and fills the electron shell of the other, resulting in two independently stable ions.
In solid, crystalline form, ionic compounds are arranged such that there is an equal distribution of the charge. Since the charges are bound to atoms, they cannot move around within the structure.
Covalent Bonds
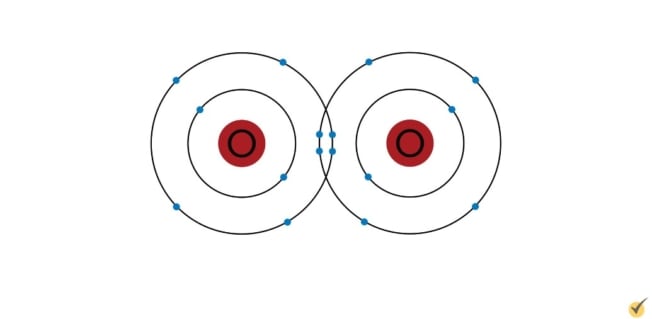
Covalent bonds typically occur between elements on the right side of the periodic table.
These elements have the same or similar electronegativities that are relatively high, as in the oxygen molecule shown.
Again, the thing of interest to us is the behavior of the electrons.
In order for each of the atoms to have a complete outer shell, the electrons of the bond are shared between them. So, the electrons are essentially bound to both atoms.
Metallic Bonds
In metallic bonds, however, the constituents are atoms of the same or similar elements with electronegativities that are relatively weak. As such, the attraction between the positive nucleus of one atom and the valence electrons of the neighboring atoms is also relatively weak.
Thus, the electrons don’t belong to a particular atom; that is, they are delocalized and are relatively free to move throughout the crystal lattice.
Electrical Conductivity
This property is the reason that metals are highly electrically conductive, malleable, and ductile.
Conductivity is the property of a material to transfer charge when placed in an electric field. If we hook up a battery to an electric circuit, the valence electrons in the material will flow in the direction of the positive terminal of the battery.
Ductility and Malleability
Ductility is a property of a metal that can be thought of as the material’s ability to be drawn or pulled. An example of this would be making a wire out of copper.
Since there aren’t discrete bonds between the atoms, metals allow for some motion of each atom about its neighbors.
It is the same reason that metals are malleable. That is, they can be deformed by being pounded. For example, gold can be pounded into sheets to make gold foil used in art.
Types of Metals and Metal Alloys
Metals on the periodic table are categorized in three main groups: alkali metals like lithium, alkaline earth metals like magnesium, and transition metals, like iron, gold, and copper.
Metal alloys like stainless steel also have metallic bonds and share similar properties. The components of the alloy are distributed throughout the structure and not bound together into a particular subunit. This is unlike covalent solids that have repeating molecules of a specific compound, or ionic solids that have a uniform repeating structure of cations and anions
Review Questions
Ok, let’s take a look at a couple of review questions before we go.
1. Which of the following properties are common in metals and alloys due to the delocalization of electrons?
- They make good insulators, have high melting points, and are poor thermal conductors
- They make good conductors and are malleable and ductile
- They make good conductors, are brittle, and have high densities
- They make good insulators and are malleable and ductile
Since the electrons are delocalized, they are available to transmit electric current. This makes them good conductors. Those free electrons also act like a glue that holds all the positive nuclei together, but since they are able to move around, the ‘glue’ is still relatively flexible. This means that the atoms can be moved around somewhat by pushing (malleable) or pulling (ductile) and still remain attached to one another.
2. True or False: Since salts, when solid, are made of ions in a crystal lattice, they are also conductive.
For salts, the ions are locked in place within the lattice and are not free to move in the presence of an electric current. However, if a salt is dissolved into a solution, then the ions are free to move and they will make that solution conductive.
That’s all for this review! Thanks for watching, and happy studying.