
Hey, guys! Welcome to this video about combustion.
You’re probably already familiar with the word from phrases like “spontaneous combustion” and “combustion engine.” You likely associate it with burning, which is great because the general definition of combustion is simply “the process of burning things.” In this video, we’re going to go beyond this simple definition and understand the chemical and physical phenomena behind combustion.
Equation for Combustion
Before we do anything else, let’s lay out the generic chemical equation for combustion and a couple of important terms:
Because different fuels can be used, there are many variants of combustion, but this is the basic formula.
Basics of a Combustion Reaction
All chemical reactions involve reactants and products. Reactants are substances that start a reaction, and products are the substances that are made by the reaction.
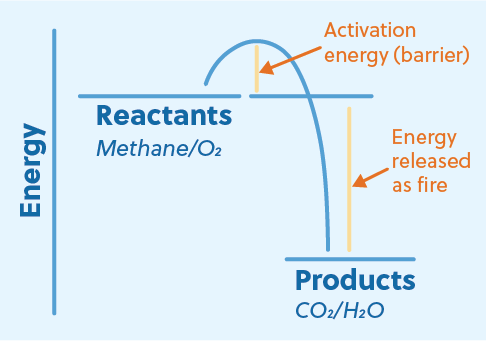
Because energy is produced, combustion is an exothermic reaction. This means that the chemical bonds of the products are more stable (lower in energy) than the chemical bonds of the reactants. When a combustion reaction occurs, the difference in energy between the reactants and products is released as a combination of heat and light, AKA fire!
Combustion Fuel
So why doesn’t firewood (a common combustion fuel) just burst into flames? After all, there’s oxygen in the air, so it seems like we have everything we need for a combustion reaction. Well, the reason firewood doesn’t spontaneously combust in the presence of oxygen is that some activation energy is required. For example, we have to use a match to start our campfires. But once a combustion reaction has started, it produces enough energy itself to overcome the barrier and self-sustain.
So what makes a good fuel for a combustion reaction? Well, looking at the generic equation, we see that oxygen is the other reactant, which gives us a clue. If the fuel reacts with oxygen, we can infer that combustion is a type of oxidation reaction. That tells us that if the fuel is going to be oxidized in the reaction, it should start out unoxidized. A common fuel in combustion reactions is hydrocarbons, like methane, ethane, and propane. These all contain simple carbon chains saturated with hydrogen. In fact, if you’ve used a gas grill, you likely used propane as a fuel in a combustion reaction to cook your food.
Methane as Combustion Fuel
Let’s look at a specific example where methane (CH₄) is the fuel.
Here, methane combines with oxygen to produce energy and two waste products, carbon dioxide and water. The reactants methane and oxygen are higher in energy than the products carbon dioxide and water. While carbon dioxide and water are both very stable compounds, the primary reason for the large energy difference is the relative instability of the single and double bonds in oxygen.
The combustion of methane is an example of complete combustion. The carbon atom in methane is completely oxidized when it is converted to carbon dioxide, resulting in the largest possible amount of energy released. However, when not enough oxygen is present, a reaction can be incomplete. This means that the carbon atoms of the reactants are not completely oxidized in the process. This not only results in less energy produced but also creates unwanted waste products that can be dangerous to the environment and human health.
Here is the chemical equation showing an example of incomplete combustion of methane:
Note that instead of producing carbon dioxide as in the complete combustion, methane is converted to carbon monoxide and carbon (soot). Because we often use air as our oxygen source, which is only 21% oxygen, incomplete combustion is more common than complete combustion.
Other Combustion Fuels
Methane is just one example of a combustion fuel. Other hydrocarbons, like ethane and propane, are also combustible. Provided enough oxygen, hydrocarbons can undergo complete combustion and are often referred to as “clean burning.” Other fuels, like wood, paper, coal, gasoline, etc., are combustible, but are more chemically complex and often undergo incomplete combustion. Therefore, the reactions are less energy-efficient and are generally worse for the environment because of toxic waste products.
Review
Okay, let’s review. Combustion is a general class of exothermic oxidation reactions where a fuel reacts with oxygen to produce waste and large amounts of energy, typically characterized by fire. Most combustion reactions require a bit of energy to overcome an initial activation barrier—why we start our gas grills with a match or spark. Most combustion reactions are incomplete, meaning that the carbon in the reactants is not completely oxidized, resulting in less energy produced and toxic waste products like carbon monoxide and soot. While the use of combustion to generate energy has been vital to the development of society, the massive amounts of waste products created pose significant threats to the environment and human health.
Thanks for watching! We hope this video on combustion leaves you prepped and empowered.
Frequently Asked Questions
Q
What is combustion?
A
The general definition of combustion is simply “the process of burning things.”
Q
What is a combustion reaction?
A
Combustion, or burning, is a sequence of chemical reactions involving fuel and an oxidant, usually oxygen, that produces heat and sometimes light.
Q
What is spontaneous combustion?
A
Spontaneous combustion is a type of combustion reaction where the reactant unexpectedly ignites, without the source of a deliberate heating source.
Q
Is combustion a chemical change?
A
Combustion is a chemical change, as heat or light occurs, reactants are used up, and new products are formed.
Q
Is combustion endothermic or exothermic?
A
Because energy is produced, combustion is an exothermic reaction.
Q
What are the products of a combustion reaction?
A
The products of a combustion reaction usually include carbon dioxide and water/water vapor.