
Hi, and welcome to this video on carbohydrates!
Today, we’ll be looking at what carbohydrates are, what their purpose is, and what they’re made of.
What is a Carbohydrate?
So, what is a carbohydrate? It’s actually a type of macromolecule. Macromolecules are essentially groups of smaller molecules bonded together to carry out a specific function. There are four types of macromolecules, all of which have specific structural and functional properties that allow them to work together to carry out different cellular processes within the body.
Let’s take a closer look at these structures and see how we can use them for classification.
Structure
Carbohydrates consist of three elements: carbon, hydrogen, and oxygen. They exist in a specific ratio of one carbon atom and two hydrogen atoms for every atom of oxygen.
A simpler way to represent this is with the formula \((CH_2O)-n\) where the \(n\) stands for any given number of these carbohydrate units. These simple sugars can link together to form more complex molecules called polymers. So, carbohydrate monomers are the subunits of carbohydrate polymers.
We can have simple sugars of just one monomer or more complex sugars with lots of monomers all bonded together. This means they vary in structure and are therefore really diverse.
Classification
One way we can classify carbohydrates is by the number of monomers they contain.
If the carbohydrate is made up of a single-sugar monomer, it is called a monosaccharide–mono, meaning “one,” and sacchar, meaning “sugar.” Each of these are made up of chains of carbon, hydrogen, and oxygen in the \((CH_2O)-n\) ratio, but they differ by \(n\).
Since \(n\) is directly related to the number of carbon atoms, we can also say they are classified by the number of carbon atoms present.
A carbohydrate with three carbons is a triose, a carbohydrate with four carbons is a tetrose, and a carbohydrate with five or six carbons is known as a pentose and a hexose respectively. These monomers exist as a ring structure, which makes them more stable.
Pentose sugars like deoxyribose and ribose make up our DNA and RNA, so we definitely don’t want those sugars to be unstable! Glucose, the primary sugar our bodies use for energy, is an example of a six-carbon sugar with the formula \(C_6H_{12}O_6\).
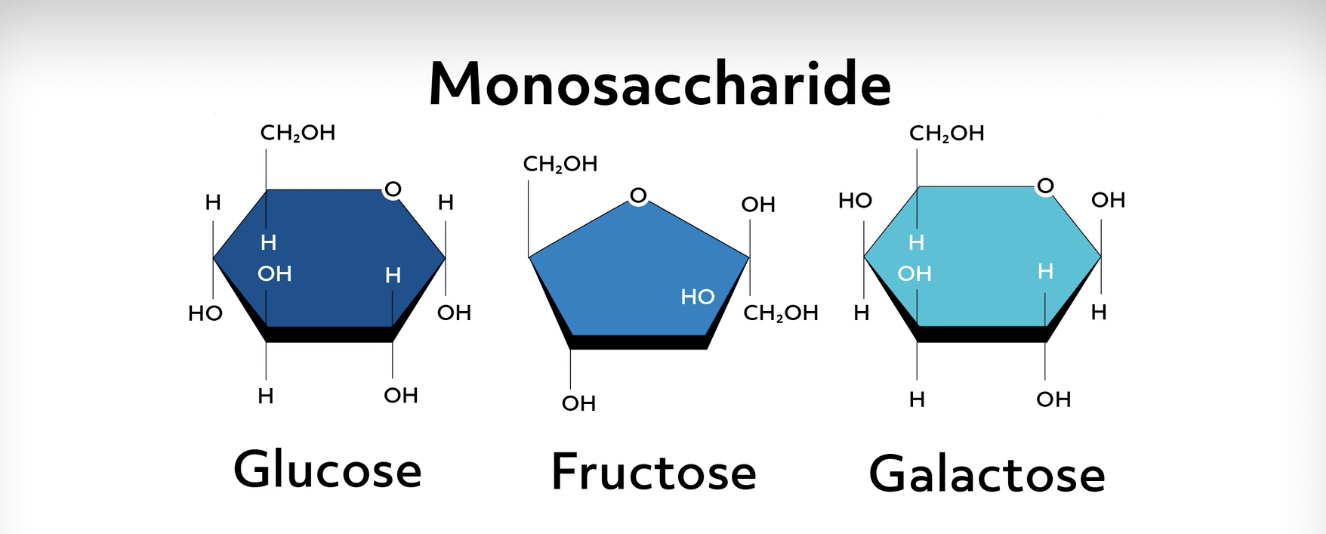
Carbohydrates can also be made up of two monomers called disaccharides. Some examples are lactose, which makes milk sweet; sucrose, which is your regular table sugar; and maltose, which is a product of starch breakdown. Each of these disaccharides is made up of two other monosaccharides.
For example, sucrose is a glucose molecule and a fructose molecule bonded together, while maltose is just two bonded glucose molecules. When carbohydrate monomers bond together, they form covalent bonds through a condensation reaction. These links are called glycosidic bonds and are named more specifically by the numeric carbon to which it bonds.
Carbohydrates that are made up of more than two monosaccharides are called oligosaccharides if they contain a few sugars (typically less than 10), and polysaccharides if they contain more than 10 sugars. So polysaccharides are just complex oligosaccharides.
Polysaccharides can be categorized based on the type of monomers they have and their structure. If a polysaccharide is made up of repeated units of the same monomer, it is called a homo-polysaccharide. Contrastingly, a polysaccharide that is made up of repeating units of different monomers is called a hetero-polysaccharide. Homo-polysaccharides and hetero-polysaccharides can exist in a simple linear, unbranched form, or a branched form depending on the monomeric chemistry.
Homopolysaccharides
Today we’ll talk about the three main homo-polysaccharides: glycogen, starch, and cellulose.
Glycogen is made up of lots of glucose monomers that glycosidically link together to form a branched structure. A branch occurs every ten glucose units of the chain. If your body has more glucose than its energy needs indicate, your body will store the glucose for later use in the form of glycogen. If your body needs to dip into its glucose store, it can easily do so by breaking glycogen down into glucose in processes like glycolysis.
Plants, on the other hand, store their glucose in the form of starch. Starch consists of a mixture of two polysaccharides: amylose or amylopectin. Amylose is a long chain of glucose monomers while amylopectin is made of branches of glucose monomers. Compared to glycogen in humans, starch is very similar; however, starch polymers are more spaced out than glycogen, meaning there’s branching less often.
Cellulose, the third form of polysaccharide, is made up of lots of long chains of glucose molecules. The glucose molecules in the individual chains are linked by glycosidic bonds like in other polysaccharides, but the chains are also bonded to each other through hydrogen bonds. Cellulose is used mostly for structural support and can be found in cell walls. Sometimes a polysaccharide called chitin is considered its own class of polysaccharide, but really it has the exact same structure as cellulose except it has an acetylated amino group at the second carbon instead of a hydroxyl group.
Carbohydrates aren’t limited to bonding just with one another. In fact, a lot of carbohydrates don’t exist solely on their own but instead form molecules called glycoproteins. Glycoproteins are found everywhere in the body and can be categorized based on their function. This dynamic duo is what gives our cells the ability to communicate with each other, gives our body a functioning immune system, and provides cushioning and support throughout.
Glycoproteins are divided up into the three following groups: glycoproteins, proteoglycans, and mucins. The glycoprotein class has a variety of functions, but a good example is erythropoietin. Erythropoietin stimulates the production of red blood cells and enhances the stability of protein in the blood. The carbohydrate portion of this molecule is what makes up the ABO antigens to give us blood types. In general, glycoproteins are proteins that bind to carbohydrates embedded in or on the outside of cell membranes. They also act as markers to help other cells identify that cell, and they can aid in cell adhesion and structural support.
The proteoglycan class is protein attached to a glycosaminoglycan. The glycosaminoglycan’s composition varies because it is made up of different repeating disaccharide units that contain amino sugars. Generally, proteoglycans are for structural support and lubrication. For example, aggrecan in the cartilage of your heel will release water molecules upon impact to provide cushion for the surrounding muscle and bone.
Whereas the glycoprotein and proteoglycan class are mostly made up of protein, the mucin class is mostly made up of carbohydrates. Mucins are responsible for hydrating cells and creating a protective barrier, so we mostly see this class of glycoprotein in mucus secretions of the respiratory, gastrointestinal, and urogenital tracts.
Ok, now that we’ve covered everything, let’s look at a couple of review questions!
Review Questions
1. Which disaccharide is made up of one glucose molecule and one fructose molecule?
- Glucose
- Sucrose
- Maltose
- Glycogen
Glucose is a monosaccharide, sucrose is made up of glucose and fructose, maltose is made up of two glucose molecules, and glycogen is a polysaccharide.
2. What kind of bond is responsible for linking sugar molecules together?
- Ionic bond
- Glycosidic bond
- Covalent bond
- Hydrogen bond
- More than one
Sugar molecules are linked together via glycosidic bonds, which are a type of covalent bond. So both B. and C. are correct. There is some hydrogen bonding that goes on between the polymer glucose chains in cellulose, so D. is also a correct answer.
That’s all for this review! Thanks for watching, and happy studying!