
The mid-1800s were a wild time to be a chemist—new elements were being discovered left and right, and we were running out of ways to keep them organized!
We needed a system, one that could organize what we knew while leaving space for what we suspected was out there.
We needed…a table.
The periodic table categorizes elements based on their chemical properties showing us how they relate to each other and helping us understand how they may react when combined. Dmitri Mendeleev is credited with the first, and perhaps most memorable, iteration of the periodic table in 1869.
So let’s dig into his work to understand why the periodic table is important and how we use it today.
What is an Atom?
First, we need to define what atoms and elements are. Atoms are one of the smallest building blocks of matter—they have a nucleus, which can have protons and neutrons, and electrons in ‘orbit’ around this nucleus.
Atoms with the same number of protons in their nucleus are said to be elements—these are the individual particles that share identical properties like density or reactivity. The number of protons in the nucleus is also called the atomic number. Think of it like a fingerprint; each element has a specific and unique atomic number.
In the early days of organization, we did not have the tools to count protons, so scientists like Mendeleev arranged the elements by increasing atomic weight. This generally worked and mostly matches what we have today. What was revolutionary about this table was that Mendeleev left blank space for elements that he was able to predict the existence of, but not prove at the time.
As our methods for discovering and analyzing atoms got better, we shifted Mendeleev’s table into what we see today. The premise is simple: list the elements in order from least number of protons to most number of protons in rows, wrapping around once you’ve hit a noble gas like helium or neon.
Design of the Periodic Table
The purpose of the periodic table is to act as a ‘one-stop-shop’ for the most important information about all of the known elements. Scientists from all disciplines use the periodic table in their everyday work. Let’s take a look at a specific element and how its place in the periodic table helps us gain information about that element.
Each element in the table contains, generally, the element’s symbol, name, and atomic number, which is the number of protons. Remember that this is what makes carbon, carbon. Any atom with exactly 6 protons in the nucleus is carbon. Any scientist investigating an unknown element will immediately look to determine the number of protons it has, so that they can identify it. Below the element’s name will be the average atomic mass, which is, as it sounds, the average mass of a carbon atom.
Other information is usually included in the box, but this can depend on what the table is being used for. For example, this symbol includes the 2,4 to indicate in which energy level carbon’s valence electrons are being stored. This is important for scientists looking to investigate how electrons move up and down energy levels in atoms.
In other cases, it may be more useful to have the electronegativity included, especially when discussing atomic bonding.
Each box on the table contains a wealth of information about each element, laid out in a way that condenses the overall footprint of the table to something that can be printed on a standard piece of paper….mostly. We’ll come back to that fact.
First let’s quickly take a look at just how many other bits of information are coded into the periodic table.
Each column highlighted here contains the same number of valence electrons, which leads to them having very similar chemical behaviors!
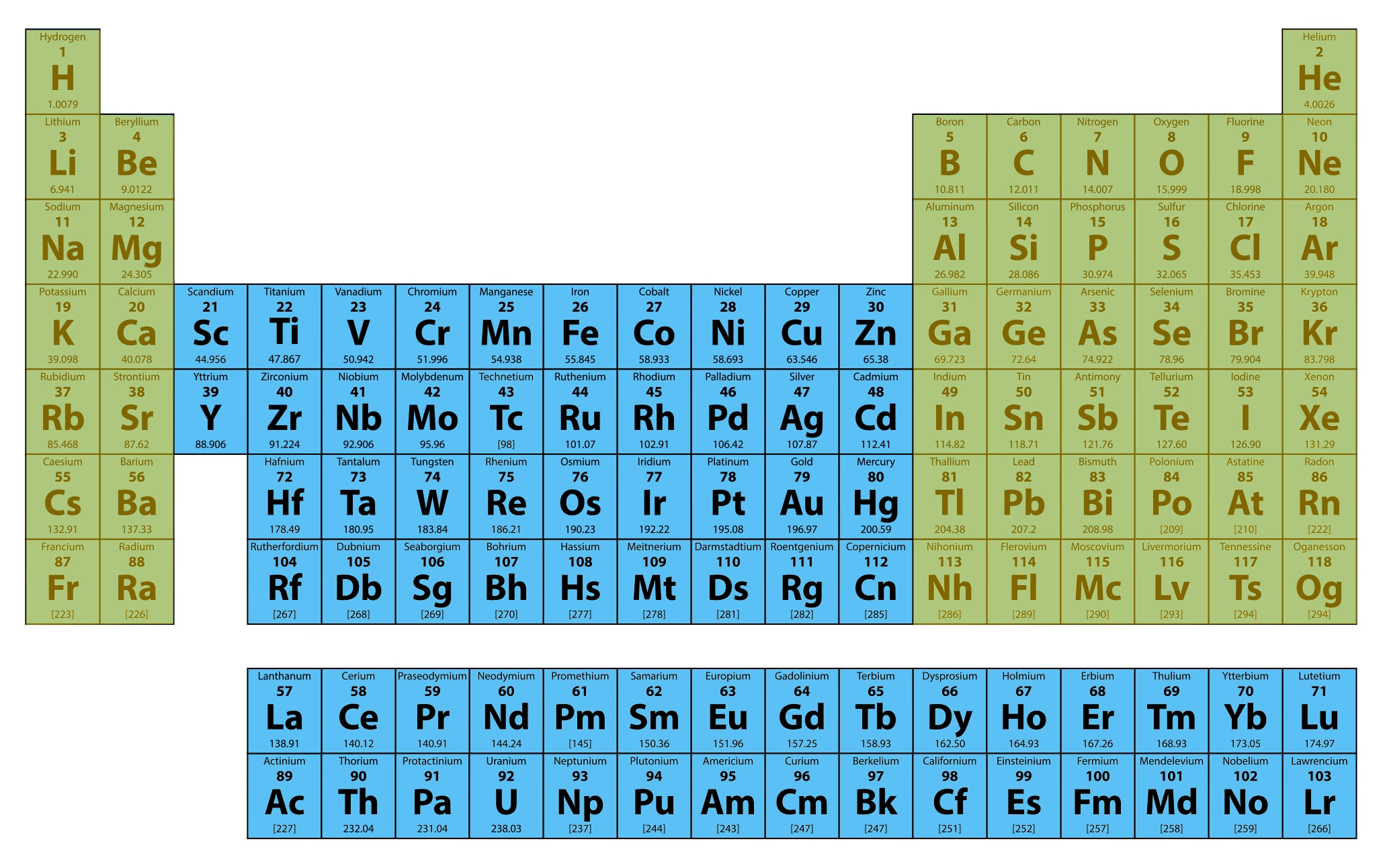
The transition metals, which make up the middle portion of the table, have some strange ways of filling energy levels with electrons. This creates some physical properties that make many transition metals important for many aspects of our technological world! For example, metals like nickel and cobalt are key components in rechargeable batteries.
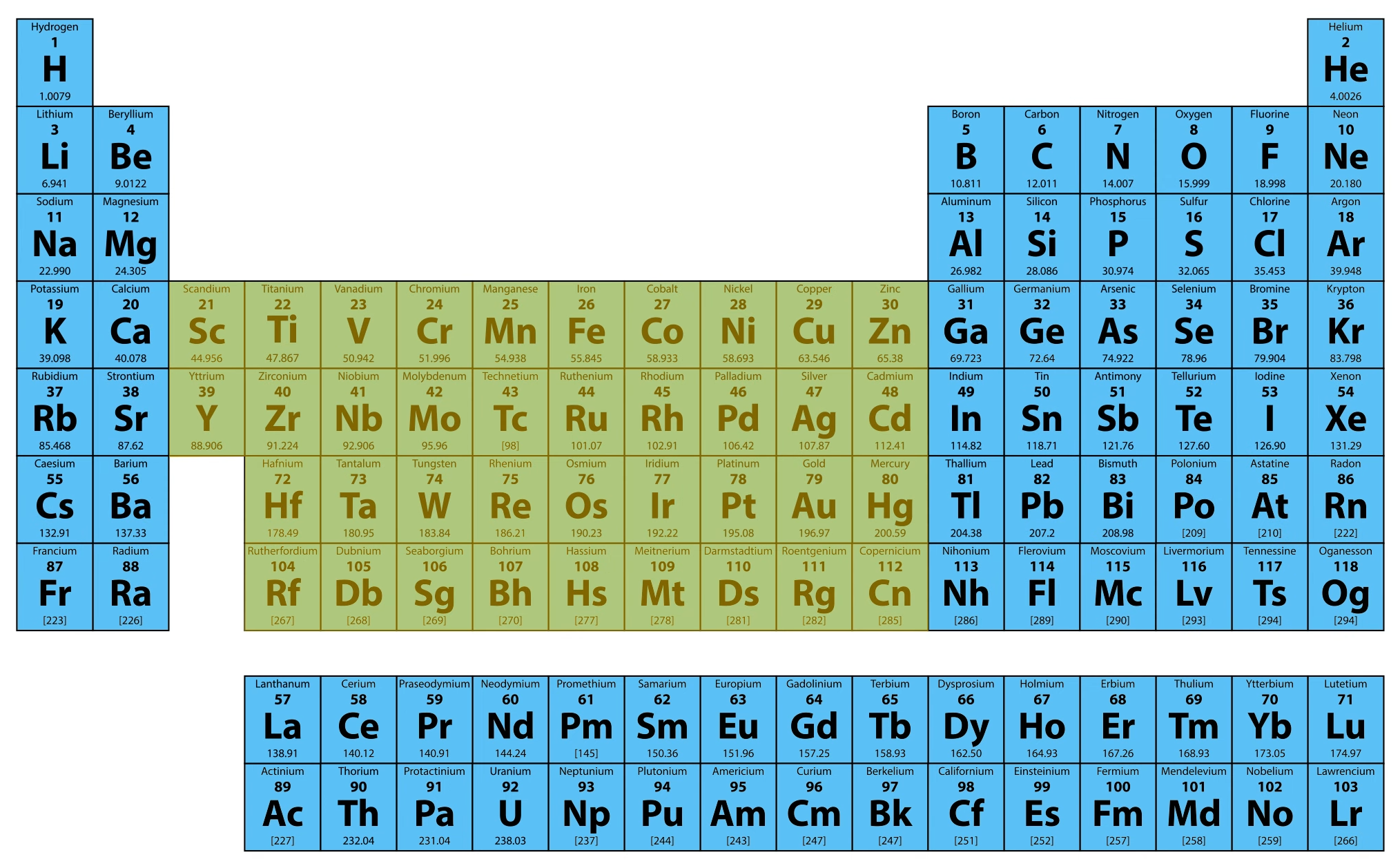
Now let’s come back to the part where we said the table could fit on one printable page. This is mostly true, but there are two rows that are cut out and placed below the table to keep it from being too wide. These are called the lanthanides and actinides.
So there you have it! The periodic table in all its glory!
Recent Additions
And we’re still adding to it today! Since the year 2000, we have discovered and added 5 new elements to the periodic table, whose existence and properties were predicted thanks to the structure of the periodic table.
These elements can only be created by smashing other, more stable elements together at high enough speeds to allow the nuclei to stick together.
The most recent element added was Oganesson, which was first created in 2002 and formally added to the table in 2016. It is so unstable that only 5 atoms of the element have ever been created, and all of them decayed in less than a millisecond.
Review
To sum up—the earliest versions of the periodic table represented a dramatic shift in how we organized elements and matter. Mendeleev’s work allowed for the prediction of undiscovered elements, and reorganizing by atomic mass further allowed for the grouping and organization of similar elements. Scientists everywhere use the periodic table as a ‘one-stop-shop’ for comparing elements and predicting chemical behavior. We are still finding new elements today, largely through the use of high-tech supercolliders!
Who knows what elements we may create next!
That’s all for this review. Thanks for watching, and happy studying!