
Hi, and welcome to this video on alkynes!
Hydrocarbon Structures
Alkynes are unsaturated hydrocarbons where some of the hydrogens have been replaced by triple carbon-carbon bonds. Thus, like alkenes, some of the hydrogens have been replaced by pi bonds between carbons.
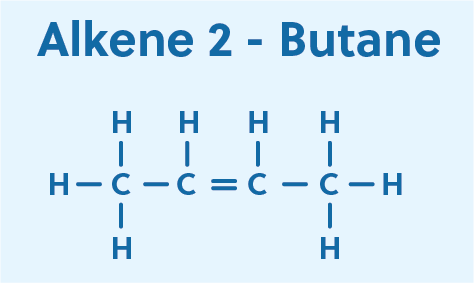
Let’s look at a simple example, starting with the alkene 2-butene and converting it to the alkyne, 2-butyne.
In 2-butene, two of the hydrogens of butane have already been replaced by a carbon-carbon pi bond. In 2-butyne, the remaining interior hydrogens are replaced with a second carbon-carbon pi bond, resulting in a triple bond between the two interior carbons.
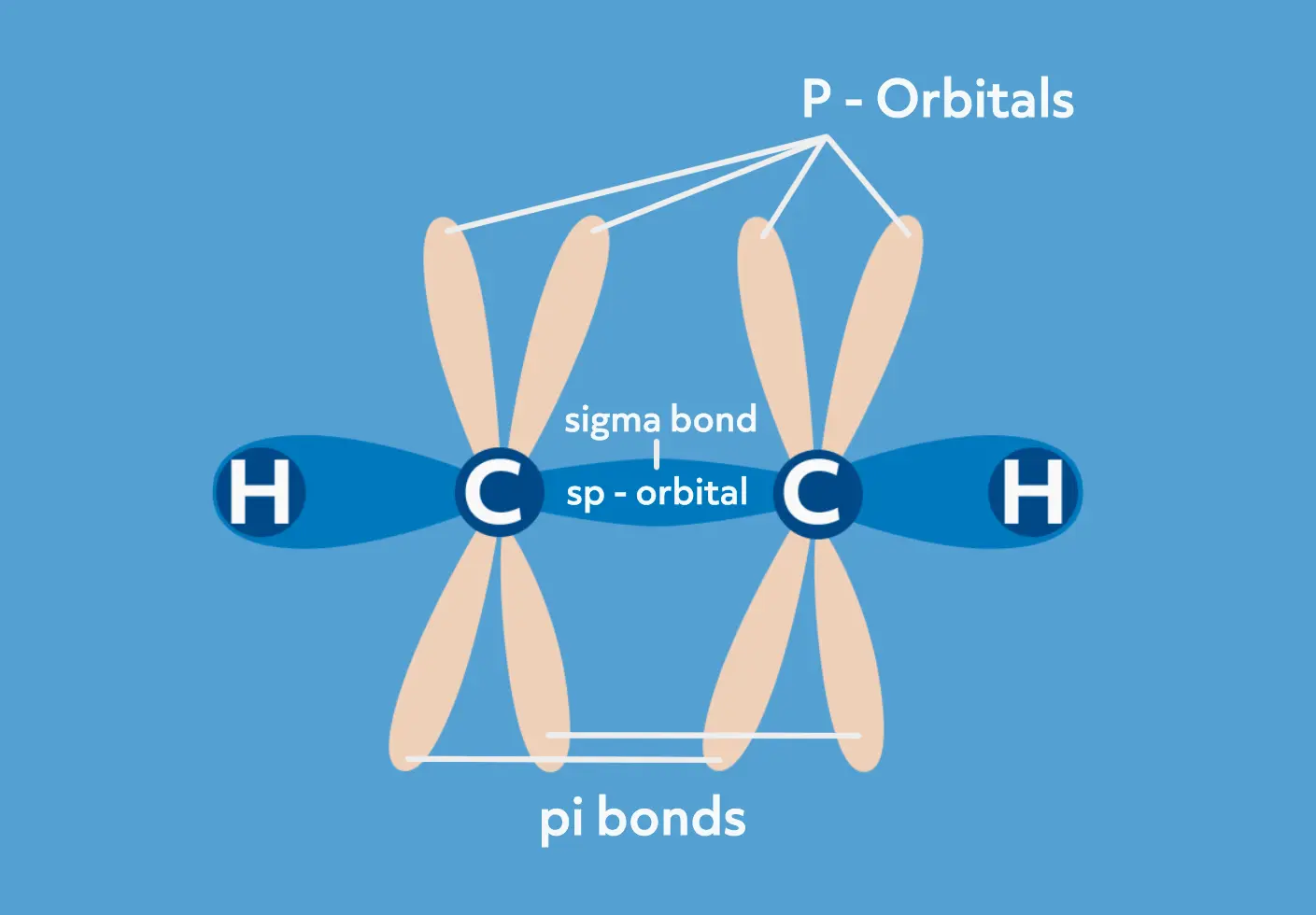
The carbons in the triple bond are sp hybridized and have a linear geometry. The triple bond is made of one sigma bond and two pi bonds. The pi bonds are made from overlapping p orbitals on each carbon, which are required to remain in the same plane. The carbon triple bond is even stronger than the carbon double bond and the bond length is shorter as well.
The simplest alkyne is acetylene, which is made of two triple bonded carbons with two hydrogens. It is a highly combustible colorless gas.
Alkynes can also have much larger carbon frames with more complex branching.
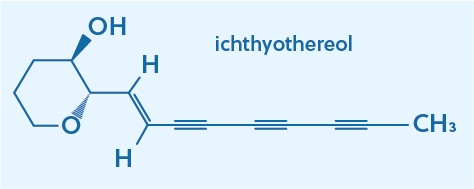
While by strict definition, an alkyne is an unsaturated hydrocarbon with at least one triple bond, it is common to describe the triple bond itself as an alkyne. So while the overall molecule might be more complex than a hydrocarbon, you can still refer to a carbon triple bond as an alkyne. For example, ichthyothereol, which is a naturally occurring poison found in several plants, has three alkyne functional groups on its hydrocarbon tail.
Properties of Alkynes
Now that we’ve learned about the structure of alkynes, let’s consider their properties. Alkynes are generally more reactive than alkenes and much more reactive than alkanes. Because they are less saturated (have fewer hydrogens) than both alkenes and alkanes, it’s possible to add more functionality to the carbon framework. For example, the two pi bonds of an alkyne can be replaced with 4 bromines in this addition reaction.
Consequently, alkynes are very useful in organic synthesis because they provide the underlying carbon framework but react readily, thus making the addition of further functionality easier to accomplish.
A point of confusion often arises here. We previously stated that the carbon-carbon triple bond is stronger than the carbon-carbon double bond (and though we didn’t explicitly say it, the carbon-carbon single bond as well). If triple bonds are stronger than double and single bonds, why are they more reactive?
The answer to this is pretty simple. The strength of the triple bond is the sum of the sigma bond plus the two pi bonds. This is, of course, a larger number than the sum of a sigma bond and a single pi bond (the double bond) and a single sigma bond. But while the sum of the bond strengths is larger for a triple bond, the individual pi bonds are weaker than the sigma bond. When alkynes (and alkenes, for that matter) react, it’s the pi bonds that break, not the sigma bonds. So even though alkynes have a greater overall bond strength, the pi bonds are individually weaker and react more readily.
Review
Let’s finish up with a quick review of what we’ve covered today. We first defined an alkyne as an unsaturated hydrocarbon with at least one carbon triple bond. We then inspected the geometry of the triple bond as well as the bonding orbitals. Lastly, we considered the properties of alkynes and why they are so reactive and thus, so useful in organic synthesis!
Thanks for watching, and happy studying!