
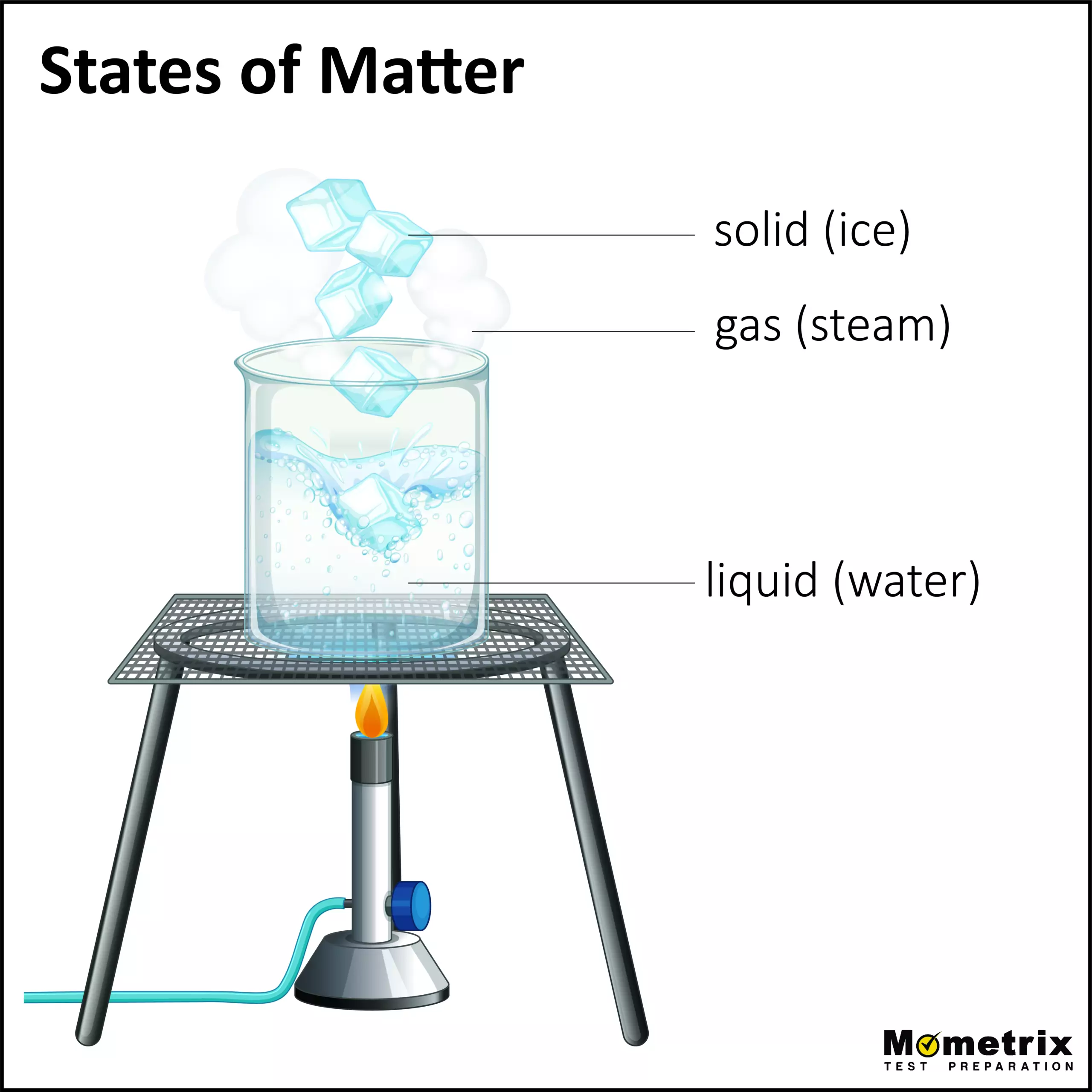
Have you ever put an ice cube in a heated skillet, and watch it transform from ice, to water, then to evaporate out of the pan and make steam? What you’re seeing is that piece of matter changing in its state.
There are three primary states that matter exists in: gas, liquid, and solid.
However, in very extreme cases matter can exist in other states, like plasma—more specifically, hot plasma.
Gas
Within a gas, particles have large gaps of space, and there is no specific pattern to their arrangements. The particles move very quickly and freely. Since there is a lot of space between particles, gas is compressible. Gas can easily flow since particles dart past each other, and gas takes the shape and volume of the container that it’s in, since the particles move freely.
Liquid
Particles within a liquid are much closer together, but they also do not have a specific arranged pattern. These liquid particles move, but not quite like gas particles. They vibrate, slowly move about, and they can also move past each other. Because the particles can move, a liquid can fill the shape of the container it’s in. However, it will not fill the whole space like gas, depending on the volume of water to volume of container ratio. Since there is not as much space between the particles, liquid is not easily compressible. Liquid can flow pretty effortlessly, which is due to the particles’ ability to slide and move past each other.
Solid
Now, particles within a solid are very, very close together with very little, if any, space between each other; and they are generally arranged in a specific pattern. Typically, solids do not move about, but they will vibrate in place. A solid has an already fixed volume and shape, since there are little to no major movements of the particles. A solid is also not easy to compress, because of the lack of space between the particles already. Solids do not flow easily, because of the lack of movement of the particles and their inability to move past each other.
Now, plasma. I mentioned that this only happens in extreme cases. Plasma is an extremely hot gas that is ionized. Plasma has significantly different characteristics than the other 3 states of matter, so it is thought of as the very distinct fourth state of matter. However, it is closest in similarity to that of a gas.
Having an understanding of what’s going on with the particles within matter is very important. They are not just simply tiny pieces of solid or liquid or gas, they are molecules and atoms. What is happening with those molecules and atoms, their physical characteristics, is what decides their state.
I hope that this video on states of matter was helpful. See you guys next time!
Frequently Asked Questions
Q
What are the states of matter?
A
The current definition of matter describes it as having four states: solid, liquid, gas, and plasma.
Q
Which state of matter is fire?
A
Fire is considered plasma.
Q
What is the state of matter of the mantle?
A
The state of matter of the Earth’s mantle is predominantly solid.
Q
Which state of matter has the most kinetic energy?
A
Plasma is the state of matter with the most kinetic energy.
