
Hi and welcome to this video about standard temperature and pressure!
In this video, we’ll review what pressure and temperature are and discuss what scientists and engineers refer to as standard temperature and pressure.
Let’s get started!
Defining Pressure
Even if you aren’t familiar with the exact formal definition of pressure, you probably have a good idea of the concept. When something pushes on your skin, you feel pressure. When you apply enough pressure to a filled balloon, it pops. But what exactly is pressure?
Pressure is defined as the amount of force applied per unit area. This should be pretty intuitive if we look at the example of something pushing on your skin. The object, let’s say a fingertip, applies a force to the skin on your arm and only does this over a certain area of skin equal to the size of the fingertip, applying pressure.
It’s important to note that pressure is a scalar quantity and is not defined as having a direction. There are a couple of different equations for pressure. The first is simply the definition of force (F) per area (A):
It may not be as obvious, but gases can also exert pressure. For gases, pressure is discussed in terms of temperature, volume, and the number of gas particles, and for this, we use the ideal gas law:
In this equation, \(P\) is the pressure, \(V\) is the volume, \(T\) is the temperature, \(R\) is the ideal gas constant, and n is the amount of gas in units of moles.
Atmospheric Pressure
An example of gas exerting pressure is the pressure from our atmosphere, referred to as atmospheric pressure. Our bodies are used to and even rely on this kind of pressure, so we don’t feel it in the way we would if something were pushing on our skin. Atmospheric pressure exists because the gravity from Earth holds down various gases that make up the atmosphere. This pressure increases the deeper you go below sea level because the amount of air above you increases.
At sea level, we say that there is 1 atm of pressure. However, as we climb up to higher and higher ground, we experience less and less atmospheric pressure because there is less and less air above us. This change in pressure is why our ears pop with changing altitudes.
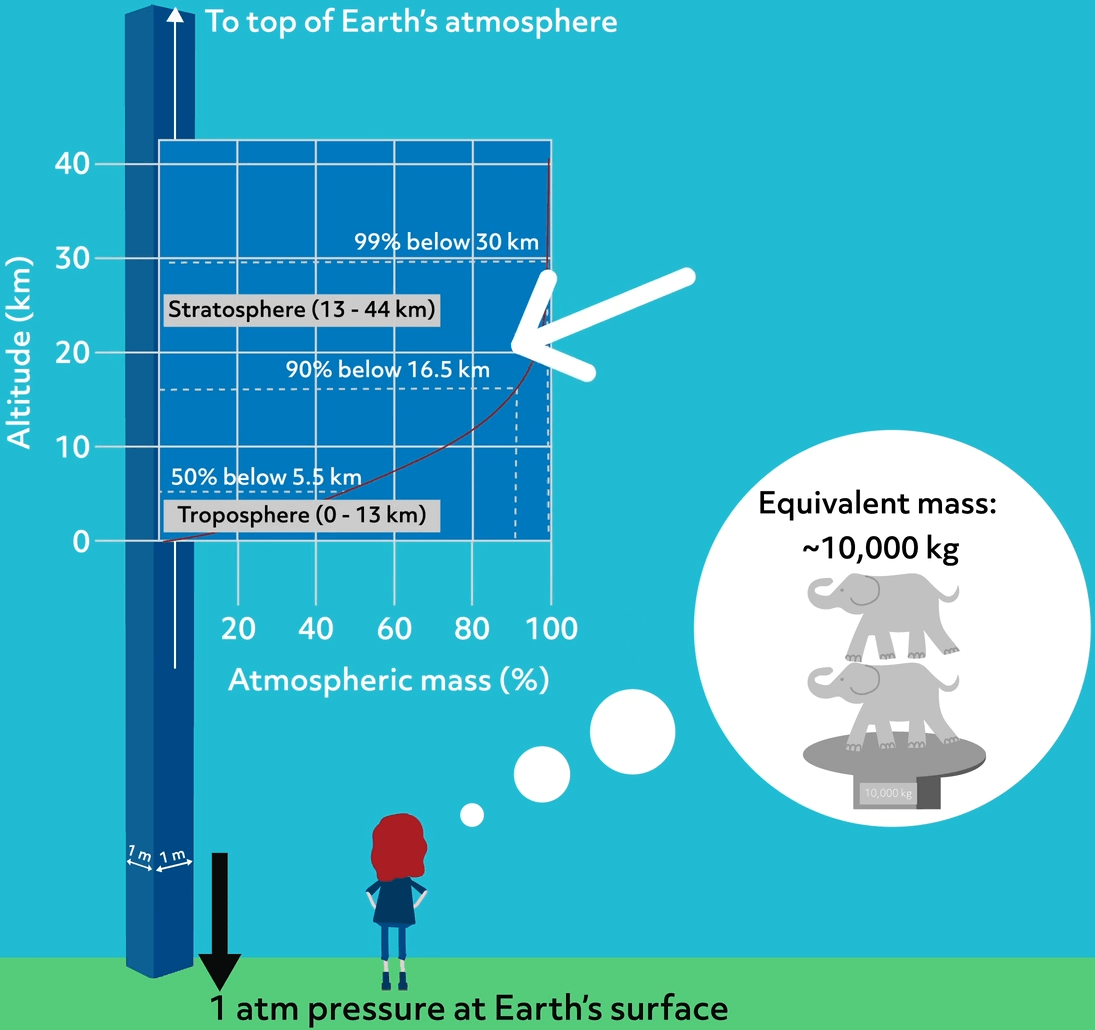
1 atm of pressure is equivalent to about a 10,000 kg mass pushing down on a square meter area. We can think of all that mass existing in a very tall and thin column above the surface.
The atmosphere is denser near the surface and thins as the distance from the Earth increases. In this image, we see that 50% of the atmospheric gases exist within 5.5 km and 90% within the first 16.5 km. Above that, gases become very thin, and due to less available oxygen, it becomes harder to breathe.
Now let’s go the opposite direction. Water is fairly heavy and there is a notable increase in pressure as you go deeper and deeper under the water. In fact, for about every 10 meters you go down, the pressure above you increases by about 1 atm.
Measuring Kinetic Energy
Now that we’ve got a better understanding of what pressure is, let’s talk about temperature. We all know that temperature measures the hotness or coldness of something, but how?
Temperature is a measurement of the kinetic energy of atoms or molecules in an object. So, when we are measuring temperature, we are measuring the amount of energy in the molecules of the object. As the object cools, it loses this heat energy to its surroundings.
Most everyday thermometers give a temperature in units of degrees Fahrenheit or degrees Celsius. However, the SI unit of temperature is Kelvin. Most scientists use this unit for temperature in their models and equations. It is a convenient unit, in part, because absolute zero (that is the lowest possible temperature) is 0 K. At 0 K, the atoms or molecules of an object are completely still.
When two objects of different temperatures come into contact, the higher energy particles (the hotter particles) will give energy to the lower energy particles until they all reach thermal equilibrium.
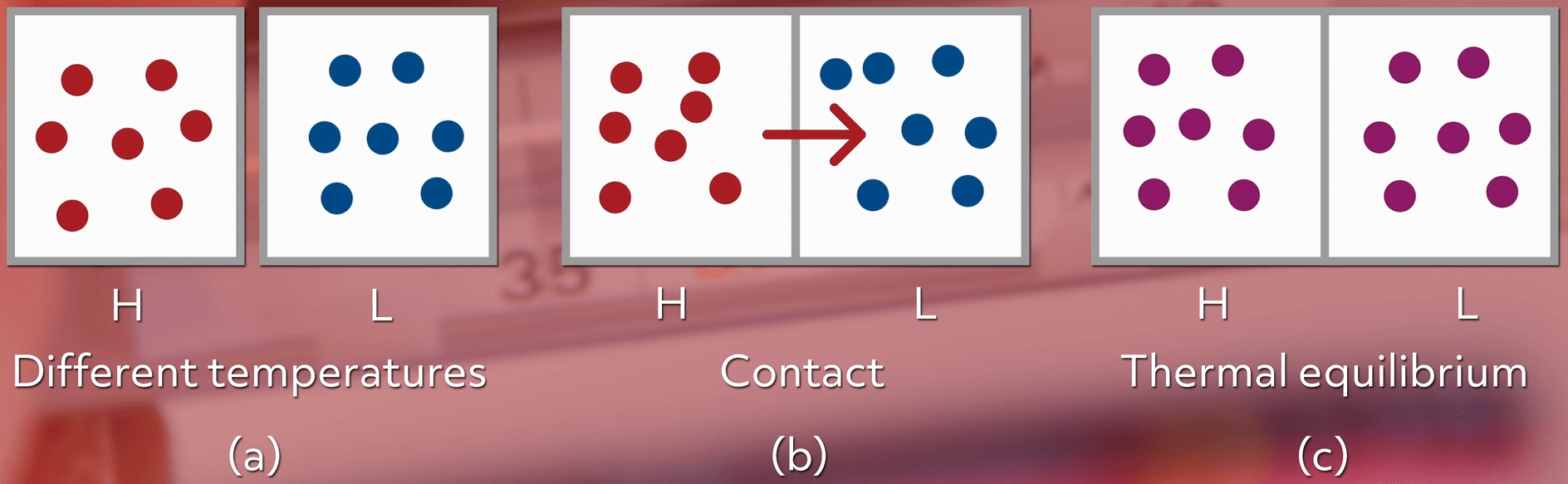
In other words, the heat will spread out evenly until the two objects are at the same temperature. An example of this would be when your cold hands are holding a hot drink. Over time, the heat from the drink will warm up your hands and the drink will cool off.
Scientists and engineers often use a set of conditions referred to as standard temperature and pressure or STP. Standard temperature is defined as 0 degrees Celsius (32 degrees Fahrenheit or 273.15 K) and standard pressure is defined as 1 atm of pressure.
These values are used to describe things like properties of different gases. Since some properties can change with temperature and pressure, it is useful to have a standard temperature and pressure with which to compare these properties.
Review Questions
Now that we’ve learned all about temperature and pressure, let’s check our understanding with some questions!
1. If you have a certain amount of an ideal gas contained in a rigid box (the volume can’t change), and then you raise the temperature inside the box, what will happen to the pressure in the box?
- The pressure will increase
- The pressure will decrease
- The pressure will not change
If we look at the equation \(PV = nRT\), then we can see that when the volume and amount of gas are constant and the temperature is raised, then the pressure must also increase. If one side of the equation increases, the other side must also increase here. Note that temperature and pressure are dependent on each other.
2. If we assume the gases that compose the atmosphere are all contained within an altitude of 44 km, which statement most accurately describes the pressure at an altitude of 22 km?
- The pressure is 0.5 atm.
- The pressure is higher than 0.5 atm.
- The pressure is lower than 0.5 atm.
The majority of the mass of our atmosphere is contained within the first 20 km of the atmosphere and the rest is quite thin, so by the time we get to 22 km, the pressure will be much less than half of the total. Less mass above you equals less pressure.
That’s all for our video on standard temperature and pressure! Thanks for watching and happy studying!